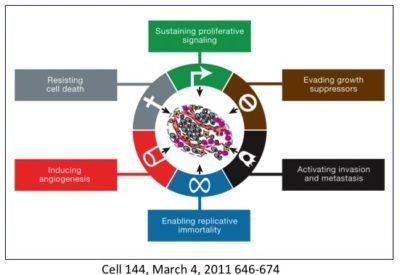
To understand cancer as a whole, rather than as individual cancers, it is useful to find out those traits that are common to all cancers. One of the most widely cited papers in oncology is ' Hallmarks of Cancer ' which initially listed 6 hallmarks and then updated in 2011 with two more.
This is a crucial step to defining cancer's common features - these 8 hallmarks. Despite cancer's many hundreds of different mutations, all cancers share these commonalties so, these must be critical features for survival that are being naturally selected as noted in ' Darwin's theory of evolution or why cancer is not simply a result of random mutations '. So, how are all cancers are similar?
First, we know that cancer cells are derived from originally normal cells. Breast cancer is derived from normal breast tissue and retains some its features, like estrogen receptors. Prostate cancer cells are derived from normal prostate cells and retain some of those features like sensitivity to hormones like testosterone. That is why castration (medical or surgical) is used in the treatment of prostate cancer and anti-estrogens like Tamoxifen are effective.
But something happens along the way to these originally normal cells that turn them cancerous and all share certain traits. Originally 6 were described and then 2 more were added in 2011. Also noted was that cancers are not a simple giant glob of a single type of cell. Instead, cancers are complex masses with multiple distinct cell types within it.
1. Sustaining proliferative signalling
There are normal genes that increase growth (onco-genes - accelerator) and decrease growth (tumor suppressor genes - brakes). In the normal situation it maintains the steady state, and when they become dysregulated, excessive growth may result (stepping on the accelerator or taking the foot off the brakes). Many such genetic mutations have been discovered, but the fundamental question remains - why are they mutated? Was is just a random accident? That's what we used to think - everything was just an accident. However, the remarkable similarity of all cancers suggests that this is no random occurrence. That is, why should all cells eventually decide to continue growing instead of say, sending out light like a firefly? It's too much to believe that this is all coincidence.
In addition to genetic factors, signals from neighbouring cells also plays a role in determining the growth of cells (tissue organization theory). That is, a stem cell located near other liver cells may become a liver cell. But such cell to cell signalling is difficult to measure experimentally and thus poorly understood. By itself, the cell is able to defend against this excessive growth by triggering senescence or apoptosis. That is, cells are not immortal - they only last a certain time. Just the like engine that is revved too much, it breaks down. Cells that constantly divide eventually become old and die.
2. Evading growth suppressors
Tumor suppressor genes act as brakes to stop growth of normal cells, as well as tumours. In order to keep growing, cancer must outwit these genes or knock them out. In addition, when growing cells in a culture, cells do not continually grow. This is known as contact inhibition. When a population of cells becomes large, it acts to suppress any further growth.
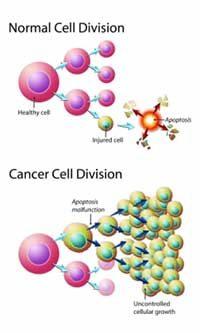
3. Resisting cell death
Programmed cell death is also known as the phenomenon of apoptosis. Under certain conditions, the cell receives a signal that certain cells should die off. The most well studied is the DNA damage sensor that operated via the TP53 tumor suppressor which then induces apoptosis. Cells with DNA damage die off and the cellular parts reclaimed. Tumors find ways around this apoptosis, most commonly by evolving mutations to the TP53 pathway which inactivates it.
There are many similarities between the pathways apoptosis and autophagy - the cellular recycling process of sub cellular parts and organelles. Importantly, autophagy has both good and bad effects. While autophagy may potentially delay the onset of cancer, once established, it may enhance cancer survival by putting it into a dormant state.
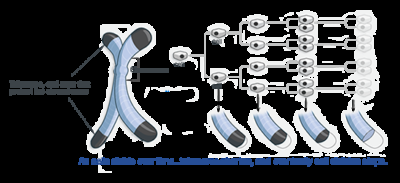
4. Enabling replicative immortality
Cancer cells are immortal. Normal cells can only replicate a certain number of times before they die. If cancer wasn't immortal, it wouldn't be a big problem. We'd only have to wait for them to die off. But they don't. Telomeres protecting the end of chromosomes are crucially important in developing immortality. Regular cells have telomeres that progressively get shorter the more times they divide. Thus, over time, as telomeres shorten, cells get old. Telomerase is an enzyme that adds more telomeres to the chromosomes. Normal cells don't have it, and immortal cells, including cancer cells, do. This blocks aging (senescence) and apoptosis.
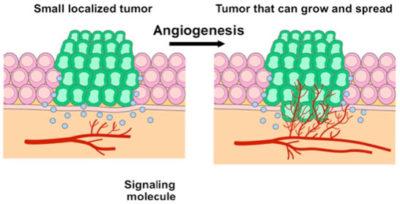
5. Inducing angiogenesis
As the cancer grows, it requires blood vessels to bring nutrients into the centre of the tumor and to remove waste products. Without acquiring this ability to grow new blood vessels, tumours would die. This led to the development of a number of drugs that targeted and blocked specific receptors in this pathway. Optimism was high that these drugs would be able to stop multiple cancers cold. Unfortunately, these drugs were minimally effective at best. The cancer eventually just found a way around the particular pathway being blocked.
6. Activating invasion and metastasis
Cancer metastasizes. This means that it moves from its place of origin to distant shores. For example, breast cancer, if it remains in the breast would be fantastically easy to treat. You simply cut off the breast, and it's done. This does not always work because very often, in advanced disease, the breast cancer has moved out of the original breast into the liver, bones and brain. These metastases are responsible for virtually all the deaths associated with cancer. Cancers that cannot move around are called benign, because they are so easily treated. Lipomas, for example, excessive growth of fat tissue, is more a nuisance than a real disease, because they do not metastasize.
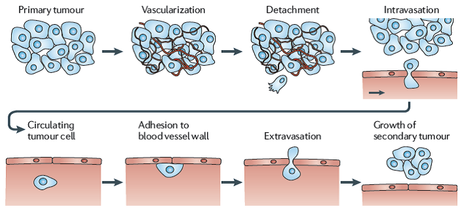
Of all the things that cancers do, the ability to metastasize is perhaps the most difficult. It involves multiple steps. Cancer cells must break free of their surrounding structure. Breast cells for example are held together by adhesion molecules, which is why you don't find normal breast cells in the lung. Then these breast cells must set up in an entirely foreign environment. Breast cancer, for example often metastasizes to the bone. But the environment of the bone is completely different from that of the breast. It's like humans trying to walk out onto the surface of Mars and expecting to flourish.
So these metastatic cells must bust out of their original tissue, somehow evade all the cells trying to kill it and then set up a new colony in an entirely alien environment and then thrive. This means the cells must now develop an entirely new set of mutations in order to survive.
Classically, metastasis is something that happens late in the course of cancer, so it was assumed that the cancer stayed intact until it had been around awhile. However, newer evidence suggests that micro-metastases may be shed from the original cancer early on but these sloughed off cells simply do not survive. It is also possible that these micro metastases survive in a dormant state. This may render them relatively impervious to standard chemotherapy drugs, which kill actively dividing cells.
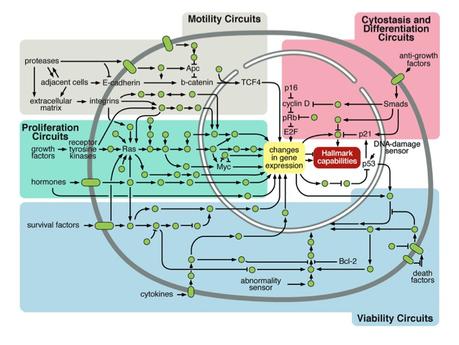
These are the original 6 Hallmarks of Cancer. When you break it down, it comes down to these main points about cancer - all of which came from an originally normal cell.
This was the state of the art in 2001, and it was a great start, but it didn't tell us anything about why all these characteristics were being selected for. Unfortunately, researchers, instead of looking at both the ' seed and soil ', decided that it was just random luck that every breast cancer in the world looked like each other, even though genetically, they were completely different. That is, all the best cancer minds in the world thought that everything about cancer could be explained by "a few hundred random mutations in gene expression that all happened to look and act exactly the same". Not impressed. That might explain why so little progress has happened in cancer biology.
But it did set the stage for others to wonder why all cancers looked the same. They grow. They are immortal. They move around. It reminds me of something. There are other cells that are exactly like this. But what are these cells? Reaching back into the mists of time, they look almost exactly like primordial single celled organisms. Whaaat? This cancer story is getter more bizarre by the minute. Stay tuned.

