A 2018 Year end Review of Scientific Research
Authored by
Alycia Halladay, PhD, CSO of the Autism Science Foundation and the
Scientific Advisory Board of the Autism Science Foundation with sincere thanks to
Cheryl Cohen at the Interactive Autism Network
Researchers have known for some time that individuals with autism spectrum disorder (ASD) and their family members show increased rates of psychiatric conditions including anxiety, depression, and attention deficit hyperactivity disorder (ADHD). A staggering one third of children with ASD also have those one or more of those disorders.1 This year’s research has given the community a deeper understanding of the biological connections between these conditions, shared genetics, and why ASD may be part of a larger spectrum of psychiatric issues affecting children and adults.
Crossover in diagnosis and in genetic profiles
Until the recent changes in the Diagnostic and Statistical Manual(DSM) (a psychiatric handbook used by doctors, therapists, and others in the U.S. to diagnose mental, behavioral, and developmental disorders), 1a person could not receive a diagnosis of both ASD and ADHD, so the clinician normally chose one or the other. Researchers only focused on the presence of ADHD orASD in families, rather than the crossover. Recently, researchers looked at first-degree relatives (parents, siblings, and children) and found a higher rate of ASD among those with ADHD and ASD2as well as bipolar disorder3and schizophrenia4. A recent study that looked at all of the previous studies of anxiety that occurred alongside of ASD showed that adults with ASD were twenty times more likely to have anxiety and much more likely to have obsessive compulsive disorder (OCD) than people without ASD7. Evidence from young children suggest that there is great overlap in early predictors of anxiety and autism, which may diverge as children get older, forming two separate disorders8. By focusing in on those with co-occurring diagnoses or enriched family history, scientists see distinct cognitive profiles 5and behavioral features6. This means that while there is crossover, there may also be specific subgroups of ASD formed by the presence of co-occuring psychiatric symptoms.

Anecdotally, the Autism Science Foundation often hears from family members of those with ASD wondering if a family history of a multitude of psychiatric issues had anything to do with the ASD diagnosis. The answer now is probably closer to a ‘yes’ than it was before, but that does not mean that anybody should ever feel guilty about another person’s diagnosis.
Based on these numbers, it is not surprising that several psychiatric issues, including ASD, all share common underlying genetic mechanisms. First, looking at the genetic profile of brain tissue obtained from the cortex of individuals affected by ASD, schizophrenia, bipolar depression and major depressive disorder, scientists revealed overlap in genetic expression. This overlap was highest between ASD, schizophrenia and bipolar depression13. While there is overlapping genetics, the brains of people with schizophrenia have a distinct lipid profile compared to autism, suggesting that schizophrenia, unlike autism, looks like accelerated aging in the brain.14Also, critical research from brains of people with autism compared to schizophrenia and bipolar disorder show that while gene are expressed similarly, the tiniest differences lead to different outcomes. Those with schizophrenia and bipolar depression express different isoforms, or end products of gene expression, which markedly changes what proteins are made15. These proteins are then what differentiate autism from schizophrenia.
Turning to analyses of gene function across these overlapping genes, these three disorders also showed similar impact of synaptic genes and those affecting the central nervous immune system13. Another landmark study included genetic information over 1 million people worldwide, including those with 17 different psychiatric (such as autism) and neurological (like Parkinson’s Disease) disorders. Psychiatric disorders were more similar to each other in genetic profiles than they were to neurological disorders, and while autism showed distinct genetic features, it overlapped with schizophrenia16. The sample used included mostly those with high intellectual ability and college attainment, so it is possible that some other relationships were not observed because of the heterogeneity of symptoms. Also of interest, ASD, ADHD, and schizophrenia are psychiatric issues with high genetic influence with similar trends in calculation of heritability across these three disorders 17. The differences across studies in heritability estimates are likely the result of all three of these conditions being the result of a variety of genetic and environmental factors, and differences in genetic factors within each disorder. No one gene causes schizophrenia, bipolar depression and autism, and even though there is overlap, it is unlikely that the same gene causes all three. While ASD can be considered an umbrella, it is now more likely than ever that schizophrenia, ADHD, and bipolar disorder are a larger umbrella over the autism umbrella. Scientists note that misdiagnosis occurs frequently between these disorders, so some genetic overlap is probably not surprising.
Exploiting genetic differences for new treatments
Given these findings, it seems important to include these

other disorders when researching autism to identify commonalities and differences to exploit any therapeutic successes one disorder has had. That does not mean that what works in one group is always going to work in another group, but it can begin an important scientific conversation. Differences within the autism spectrum are seen in syndromes with a high prevalence of autism. For example, people with Phelan-McDermid Syndrome, or PMS, have autism about 70% of the time18. About 75% of people with Fragile X syndrome also have ASD19. Given this overlap, researchers have started to find out which interventions work for forms of ASD with and without known causes 20-21. Because people with a syndromic form of autism are more similar to eachother than those without an established genetic link, starting from a place where there are fewer behavioral differences will provide answers, maybe even faster, than if scientists only studied those with no known established genetic link.
A new collaboration across these different rare genetic groups, called AGENDA, was formed this year. AGENDA will allow patient advocacy groups to share solutions to common problems experienced in research and by families.
One example of scientific evidence why these different rare genetic disorders overlap comes from brain tissue research. Researchers who study the brains of people with autism examined a protein, called FMRP, which is missing in people with Fragile X syndrome. They looked for this protein in those with a genetic form of autism called Dup15q Syndrome, as well as those with idiopathic autism. Idiopathic autism is a term for those with no known genetic cause of autism. The researchers found similarities between expression of FMRP in the same brain areas of those with autism and a known genetic mutation of chromosome 15 (Dup15q Syndrome), as well as idiopathic autism22. This could mean that targeting FMRP is a viable therapeutic option for all forms of ASD, regardless of genetic makeup. These findings would not be possible without the Autism BrainNet, a resource that supports the collection and analysis of brains of people with autism.

It Takes Brains
" data-orig-size="556,624" sizes="(max-width: 200px) 100vw, 200px" data-image-title="ITB Head" data-orig-file="https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=200&h;=224" data-image-meta="{"aperture":"0","credit":"","camera":"","caption":"","created_timestamp":"0","copyright":"","focal_length":"0","iso":"0","shutter_speed":"0","title":"","orientation":"0"}" width="200" data-medium-file="https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=200&h;=224?w=267" data-permalink="https://autismsciencefoundation.wordpress.com/2017/12/20/the-big-h-word-heterogeneity-in-autism-and-how-science-is-addressing-it/screen-shot-2017-12-20-at-12-55-47-pm/" alt="itb-head" height="224" srcset="https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=200&h;=224 200w, https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=400&h;=448 400w, https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=134&h;=150 134w, https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=267&h;=300 267w" class=" wp-image-3413 alignleft" data-large-file="https://autismsciencefoundation.files.wordpress.com/2017/12/screen-shot-2017-12-20-at-12-55-47-pm.png?w=200&h;=224?w=500" />To learn more about the Autism BrainNet, and importantly, to register to receive information every quarter on the progress of research thanks to studying brain tissue, go to www.takesbrains.org/signup.New prevalence estimates, old numbers
The question of “how many people have an autism diagnosis” seems to have a different answer depending on when, where, and how you ask. This year was no different. Global prevalence numbers across the world continue to be collected and analyzed and are influenced by culture, stigma, and the availability of diagnostic measures. Within the U.S., epidemiologists again saw two different prevalence numbers. The first, 1:59, was collected using the Autism and Developmental Disabilities Monitoring Network, examining educational records of those who had received a formal diagnosis24. It matches prevalence numbers from 2016. The other number came from an online or mail survey, previously a telephone survey, which asked parents whether they had a child with an ASD diagnosis. That number was reported in November 2018 as 1:4010. Different scientists have argued the advantages and disadvantages of each methodology, and they both have their strengths and weaknesses.
A prevalence study of autism diagnosis across time from Denmark might help reconcile this difference in numbers. As it turns out, prevalence of ASD rose in Denmark with age, as there are additional opportunities through school age to be identified25. In that study, the prevalence of ASD was the highest reported so far: 1:35 in 16 year olds born in 2000-2001, suggesting for the first time that prevalence may change with age. Since the ADDM study examined 8 year olds, and the 1:40 study examined children across different ranges, age may be a factor in prevalence. Additional data from the U.S. suggests that a small group of individuals who miss the diagnostic cutoff at age three, might meet it at age five26. There are some kids who teeter on a diagnosis for years. Whether or not 1:59 or 1:40 is closer to the true prevalence of autism still remains to be seen, but the MMWR does report unique: it further illustrates the racial and ethnic disparity in access to diagnosis seen in the U.S.24 More work in better identifying people with autism at different ages, and from different backgrounds, so nobody is missed, is sorely needed.
Where are the girls?

However, researchers cannot rule out a biological underpinning of a sex bias. Sex hormones (androgens) at critical areas of development can influence the function of brain circuits associated with autism, and a sex bias as a result of hormone exposure or alteration in hormone levels is possible30. During key points in brain cell development, these hormones can cause the cells to divide too quickly and affects disruptions in how cells turn into brain cells or other types of cells in the body31, which are features of cells in autism. Unfortunately, many studies looking at environmental factors that lead to autism in males and female children have either not shown a difference across sex, or not looked at sex of the child 32-34. This prevents researchers from determining whether these environmental factors result in a sex bias. The presence of the core features of autism seem to be similar for both males and females, though they may differ by severity or age. For example, in a large study conducted in the U.K., males started out with higher autistic social traits, but as adolescence approached, that was no longer the case35. Features of autism in males and females, may change over time, or get more or less severe, depending on sex.
If the core symptoms are present in females, why are fewer females diagnosed? This continues to be an important area of research. We do know that females do not escape the conditions that occur alongside ASD. Previous research has shown a higher level of internalizing behavior in females with ASD. Internalizing behaviors are those that are inwardly expressed, including depression and anxiety. Externalizing, or outwardly expressed behaviors, include acting-out behaviors such as aggression or impulsivity. This year, scientists examined another outward symptom: hoarding. Children with autism are five times more likely to show hoarding behavior . This is similar to the hoarding rates in ADHD and OCD36, with girls showing more hoarding behavior than boys do.
Genes and environment, not genes or the environment
Since environmental factors were just mentioned with regards to sex differences, it should be noted that while environmental factors may not account for the sex bias in autism, they continue to be investigated and reported as influence in an ASD diagnosis. For example, there continues to be mounting evidence that air toxins, including air pollution, produces an increased probability of having a child with autism33,34,37. Since the type and source of air pollution varies by country and region, it is not surprising that there may be some variability in the findings – with some studies showing an effect of one chemical, and others another component of air pollution. Air pollution as a whole should be of high concern across the world, and research should continue to build scientific evidence of its harmful effects, not just in autism, but also in many developmental disorders. A chemical which scientists thought was no longer a problem because it had been banned 20 years ago, was recently associated with autism38. While the effect was not large, it should serve as a reminder that known toxicants don’t always disappear when legislation changes, or manufacturing processes evolve. Other maternal health factors have been shown to influence later autism diagnosis, from polycystic ovarian syndrome in mother32, history of immune issues39, and even opioid use before conception40.
Though it is important to find factors that may contribute to autism, it is also very important to identify factors that don’t contribute to autism. This year, researchers in Scandinavia were able to dispel the theory that autism is caused by too many antibiotics given because of frequent ear infections41.
While scientists continue to make significant progress in understanding the role of genetics and genetic influence in autism risk, they were unable to better illustrate the mechanisms by which genes and the environment interact. However, new ways to study the environment in autism have emerged. Researchers were able to show the feasibility of using baby teeth to study environmental exposures during pregnancy. Because the layers of the teeth form like rings on a tree during gestation, each layer can be analyzed precisely to examine month-to-month environmental exposure or change in metabolism. This year, researchers who examined baby teeth of those with a later autism diagnosis, found that the normal cycles of zinc and copper, which occur as part of normal biological processes, were altered in ASD42. This might reflect an inability to detoxify the body following an exposure during pregnancy. However, that theory needs further study.
Also in 2018, researchers used brain tissue to show that genes relating to mitochondrial function were altered in those with autism, and the changes correlated to genes which affect how brain cells, or neurons, are shaped and function. These findings suggest that mitochondria, which are the targets of environmental factors, may act by altering the function of autism-related genes43.
It is likely that there will be an increase in the discovery of rare genetic mutations that strongly influence autism diagnosis. One type of mutation, called de novo mutations, are not seen in either parent, but are seen in the diagnosed individual. These gene changes sometimes overlap with autism, intellectual disability, and those that are involved in brain function. Right now, there are about two dozen rare de novo gene mutations accounting for about 10% of the cases of autism. With more information from whole genome sequencing, this percent may increase.
But what about the rest of autism? Is it all caused by de novo mutations that occur spontaneously? There is another type of mutation called common variation. The theory behind common variation is that autism symptoms are a spectrum, and to some degree everyone may fall on some part of the spectrum of autism traits. These traits may correspond to specific genetic signals. In most cases, autism develops from the right combination of , or too many, common variants, including known autism risk genes and those that aren’t associated with autism. As mentioned earlier, scientists now believe autism sits on the spectrum with other psychological issues and therefore, the genes associated with traits in the parents can combine, leading to increased chances that the child will receive an autism diagnosis45. Some of these are inherited, some may be de-novo, but they are not always inherited as autism genes, they can be inherited as common variations across the genome. More evidence comes from understanding the broader autism phenotype in families. Instead of looking at individuals with autism and their siblings, researchers explored genetic markers in all family members like parents, including ones with the broader autism phenotype, and found overlap in some of the genetic markers46. Even within families with lots of differences in clinical presentation of symptoms, there are some overlapping genetic factors. This demonstrates the importance of common variation in autism. However, it’s important to distinguish common variation that determines the color of our skin, to common variation which leads to autism. Common variation does not mean that genes associated with hair color can combine and lead to autism. These genes involved in common variation are known to control the way different brain regions connect with each other, how brain cells are shaped, and how they function. Common variation means that there may be different subgroups of autism caused by different subgroups of genes that can be inherited and de novo. And in autism, common variation works together with rare variation.
Now back to new genetic findings thanks to
Some promotor regions of genes show mutations in ASD. From An et al., 2018
whole genome sequencing. As explained earlier, new areas of the genome are able to be studied thanks to new genetic technologies. Two different research groups discovered this year that other areas, called non-coding regions, harbor variants that confer risk for autism47,48. Those promotor regions discussed earlier are also non-coding regions, but these studies looked at expression in family members. Using this design, geneticists calculated that the origin, while not the mutation but the origin of the mutation, came from the father’s side47and a model of combined influence of paternal and maternal factors to generate these seemingly “new” or “de novo” mutations emerged 47,48. It should be noted, however, that these findings should be viewed with caution because there are a lot of different places to look on the entire genome and scientists may need to adjust their statistical analysis techniques to make sure their findings are not by chance49. In other words, stay tuned. What geneticists continue to be excited about is the discovery of new genes that are targets of existing therapeutics and can explain co-occuring medical or psychiatric symptoms45.
A better understanding of these targets is needed, which is why animal models are so important.
Animals don’t have autism, but they can tell scientists a lot about autism

photo courtesy of Jill Silverman, UC Davis
Once scientists identify a target in the brain of someone with autism, it might take years of effort, money, and patience for that discovery to turn into something that can help people. This is a difficult wait, but it can pay off. This year, a new drug received fast track approval to treat the core symptoms of autism: balovaptan50. It targets the vasopressin receptor. It has been shown to positively influence social interaction and social communication in adults. But the road to balovaptan had to start with animal models – not just to see if it works but also to explore what dose, and what behaviors were the most promising to target in people.
These models are becoming more sophisticated, more specific, and with each model comes a better approximation of autism symptoms. For example, researchers are using rats to examine social communication deficits that better approximate conversation51. To be clear, autism is specifically human, but new therapies are becoming closer to real possibilities with the help of these models– for what works, and what does not. For example, environmental enrichment (a rough approximation of behavioral intervention in rodents) has been shown to be beneficial in some models of autism, but not so in genetic models like SHANK3 knockouts52. Parents of those whose children did not benefit from behavioral intervention see this as a possible explanation for why they haven’t seen progress. However, it does not mean that early behavioral intervention is not effective but that behavioral intervention may not work as well as in others for a variety of reasons, including genetics. These models have identified existing drugs can target autism-related behaviors in SHANK models of ASD53. These animal models can also help researchers identify similarities and differences on a cellular level between neurodevelopmental disorders, including ADHD and ASD54, narrow down the mechanism of sex differences in autism,55as well as better understand the contribution of individual genes to ASD as part of a larger group of genetic mutations, which is the case with most people with ASD56.
Another important model for autism is the use of cells
Example of groups of stem cells in a dish, called “organoids”
obtained from people with ASD. Researchers are able to turn skin cells into embryonic-like cells, called induced pluripotent stem cells, and then into brain cells, or any type of cell they want. Isn’t this totally amazing? Scientists can use skin cells and turn them into brain cells and study the function of those cells57. Induced pluripotent stem cells obtained from humans can also offer results fairly quickly, allowing scientific advances to emerge more rapidly than by obtaining and using brain tissue58. They can also allow for multiple sets of questions to be answered at once, by introducing new genes, environmental factors, or both into the mix58.
This process does not replace the important resource of studying cells directly from brains of people with autism. However, there is just not enough brain tissue to use so scientists are exploring other options. In fact, researchers use a combination of brain tissue analysis and neural stem cells to identify specific molecules in brain development in those with ASD, and the function of those molecules by inserting those molecules into neural stem cells59. It has validated the role of some small molecules in both brain development in ASD, and the continued production of cells in the brain including those that share information and those that have more support roles59. Using cells as a model for autism has huge potential for better understanding of the basic biology of ASD, which will lead to better individualized interventions.
Genetics and intermediate level phenotypes: endophenotypes
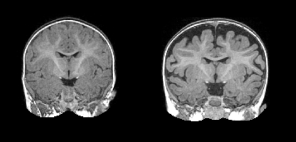
Mark Shen MRI
" data-orig-size="1894,908" sizes="(max-width: 296px) 100vw, 296px" data-image-title="MarkShen-UNC" data-orig-file="https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=296&h;=142" data-image-meta="{"aperture":"0","credit":"","camera":"","caption":"","created_timestamp":"0","copyright":"","focal_length":"0","iso":"0","shutter_speed":"0","title":"","orientation":"0"}" width="296" data-medium-file="https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=296&h;=142?w=300" data-permalink="https://autismsciencefoundation.wordpress.com/2017/12/20/the-big-h-word-heterogeneity-in-autism-and-how-science-is-addressing-it/markshen-unc/" alt="shen-mri" height="142" srcset="https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=296&h;=142 296w, https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=592&h;=284 592w, https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=150&h;=72 150w, https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=300&h;=144 300w" class="alignnone wp-image-3410" data-large-file="https://autismsciencefoundation.files.wordpress.com/2017/12/markshen-unc.png?w=296&h;=142?w=500" />Extra-axial fluid in the brain of a child with ASD (right). Credit: Mark Shen, University of North Carolina at Chapel Hill
Scientists are spending more and more time identifying the endophenotypesof autism to better understand autism subgroups. Endophenotypes are groups of biological markers that might serve in better diagnosis, but are not autism. They are usually tied to genetics. These markers are incredibly important in both understanding the development of autism and serving as objective markers for treatment protocols. They can include brain activity, brain structure, or behaviors like language delay.
This year researchers explored some of these endophenotypes not as a single biomarker, but things that could be added together to create a profile of probability of a diagnosis. The Baby Siblings Research Consortium, a group that tracks infants from high-risk families through diagnosis and adolescence, has made great advances this year. For example, early language abilities, especially understanding language, emerged as a strong endophenotype60. In fact, persistent language delay predicted ASD outcome in an independent group of kids61, reinforcing its use as an intermediate phenotype. In addition, changes in brainwave patterns, even as early as three months of age, years before a diagnosis, were shown to be a strong predictor of diagnosis62and even abnormal brainwave patterns without full blown seizure activity is common in those with ASD63. Measuring brainwave pattern through a technique called electroencephalography or EEG is viable in a number of different common clinical situations and might be used in larger scale studies besides those with high genetic risk for ASD. In addition to studying brain function, features of brain structure were also identified. Multiple replications in different samples showed a significant increase in extra axial fluid around the brains of children with autism64. This could be a marker of a biologically distinct subtype of autism. Researchers continue to study endophenotypes as markers of change, improvement, or prediction.
Better outcome measures for better treatment
While progress is being made in biological features of autism which can stratify different subgroups of autism, or measure biological differences across time through treatment to measure change, they are not yet ready for use in clinical settings. Unfortunately, the behavioral measures that scientists have used so far to measure treatment response were either developed for people without ASD, or those that were not intended to differences across time. Researchers have been working for years on developing measures which are based on clinician observation to document progress and can detect these improvements. This year, the BOSCC, or Brief Observation of Social Communication Change was validated65and is now being used in ongoing research studies. It is also important to note that this year, interventions were delivered in even a wider choice of settings. Preliminary results showed that Early Intense Behavioral Intervention, based on the principles of ABA, improved cognitive functioning and allowed for less restrictive school placements66. ABA or applied behavioral analysis, should be considered more as a set of guiding principles delivered by trained clinicians, rather than one specific practice. Importantly the interventions were delivered by trained community providers, which is where most kids and autistic adults receive help. Moving some of these interventions into the schools can be challenging, but progress was made this year. At the risk of sounding like a broken record, teachers are overworked, underpaid, sometimes unappreciated, but in most cases, they want to do what is best for their students. There can be wide variability in how teachers implement what they have learned during training into a chaotic classroom with students of varying needs. Despite this, teachers have taken on interventions that focus on things like transitions in elementary classrooms, with some success67. This has required developing outcomes based not just on child outcome, but teacher behaviors as well. Going forward, not only is there need for new measures, but also continuing partnerships from clinic to real world practice68.
Autism, employment, and suicide
Autism originates in childhood, and researchers have focused a lot of their time understanding autism in childhood. There is still much to be learned, including predicting adult outcomes based on features of autism in childhood, better understanding of the early signs and symptoms for better understanding of autism across the lifespan, the role of early intervention on longer term trajectories, and how early biological markers can help in better diagnosis and understanding of symptoms.

photo courtesy of Extraordinary Ventures
An increasing number of researchers are focusing their efforts on factors that affect the quality of life, rather than autistic symptomatology, of individuals at all ages. This year, the International Society for Autism Research provided support for the development of a policy brief on employment in people with autism, and some of the early results support the need for a strengths based approach to employing people on the spectrum69. They noted that most of the literature has used approaches to manage symptoms in the workplace or help individuals with autism reduce problem behaviors.
An alternative approach could be to identify strengths of individuals and use those strengths productively.69 In addition, based on feedback from families and individuals on the spectrum70, researchers are using employment as an outcome measure for interventions including cognitive behavioral therapy71and other therapies72.
Scientists are just starting to understand a taboo subject in autism that typically occurs in adolescence to early adulthood: suicide. A recent review revealed that most of the research on suicide in autism has been conducted in the last five years73. In one study, those with social communication deficits rather than an autism diagnosis showed a higher rate of suicidal ideation74. Social communication deficits as a core symptom of autism, could be the target of interventions to prevent suicidal ideation. In other studies, those with autism have been shown to have up to a 66% rate of suicidal ideation and 30% rate of suicide attempt75. Unfortunately, there are still no good diagnostic tools to detect suicidal thoughts in those with autism75. Specific to the autism community, unmet care needs rose as an important risk factor for suicidal ideation76. Data gathered about people who are hospitalized for psychiatric problems (psychiatric inpatients) show that depression and anxiety are important risk factors for suicidal ideation and those conditions should be treated in this population77.
Lessons from the autism inpatient collection
Psychiatric inpatients have as high as a 10% rate of an ASD78, and because of their unique features, those with severe behaviors are often the least likely to be researched79. Those with more severe behaviors tend to be underrepresented in autism research and their needs are likely misunderstood80 Their needs are often ignored, but thanks to the Autism Inpatient Collection (AIC) (https://iancommunity.org/aic), a research study which includes many of the larger autism inpatient populations, this is changing. By studying autism inpatients, researchers have the opportunity to examine issues relating to those whose minimal language, intellectual disability, and self-injury requiring hospitalization. Not surprisingly, rates of documented self-injurious behaviors are higher in a hospital setting than in a community setting because that is why patients are there. In the AIC, those who exhibited self-injury at home and in the hospital had lower IQ and more severe repetitive behavior scores, but no differences in autism severity81. On the other hand, behaviors such as irritability and hyperactivity are also associated with shorter sleep in hospital settings, which is also not influenced by autism severity scores82.
The lack of connection between autism severity, sleep, and self-injury suggests that self-injury and shorter sleep duration may represent a different dimension of autism rather than a feature of autism itself.
Treatments from plants, pills, and the classroom:
One of the hottest topics this year has been the approval

In addition to cannabis, in 2018 researchers explored additional pharmaceutical treatments. They include drugs that were previously used to treat other conditions. For example, bumetanide, a drug used to treat kidney failure, showed positive responses on eye contact in people with autism86. It also alleviated over activation of the amygdala,86which is thought to be a neurobiological feature of ASD and anxiety.
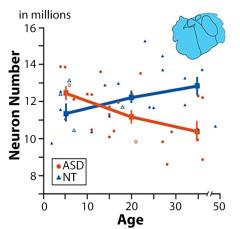
Kids with autism have more neurons in the amygdala, but by adolescence, they have fewer. Figure courtesy of Thomas Avino at UC Davis
The amygdala is known as the fear center, and ties emotional values onto different stimuli, making it a key area of interest in ASD. In fact, a groundbreaking study of autism brain tissue from individuals of multiple ages showed that the while kids with autism started out with a higher number of neurons in the amygdala, around adolescence, they dropped to lower than those who were neurotypical87. This suggests that the excess of cells in the amygdala early on cause this region to be too excited, and possibly leads to toxicity87. This important finding of a developmental shift in the amygdala in adolescence supports the amygdala as a focus for future treatments. In addition, simvastatin, a drug used to treat high cholesterol, also showed positive effects in two different studies of autism88,89.
But not all effective treatments for autism come in the form of a pill or an oil. Behavioral based interventions that were once used only for preschoolers, are now being deployed in classroom settings. Thanks to patient, supportive researchers who worked closely with dedicated, hardworking teachers, the teachers delivered behavioral interventions that resulted in improved outcomes in students67,90. These improvements were not easy – teachers must be trained and be willing to incorporate the intervention into the classroom and receive instruction.
Tristram Smith, one of the researchers involved in a study of kids in low resourced schools,67passed away in 2018. His compassion and dedication to the field is sorely missed. He was crucial in helping to show that teachers and interventionists who work together can make a difference for school-aged kids with ASD.
Through the years
If money and time were no object, there would be more longitudinal studies in ASD. Longitudinal studies in autism track each person on an individual basis from before they received a diagnosis through adulthood. Longitudinal research is able to not just look at differences between those with autism and without, but also look at the trajectories of behavior across a few years to a few decades. These studies can describe features of autism or be predictive in helping identify subgroups that respond to treatment or identify early features to predict later ones. A collaboration between multiple research groups has tracked a group of people with autism from age 2 to now age 19 and identified common and distinct patterns of behavioral development, illustrating what can happen as children become adults. It shows that, overall, there is improvement in some symptoms of autism including social communication. However, this improvement was somewhat dependent on language ability91. While this is not always the case, it shows that there are always lifelong opportunities to learn, and improvements can be seen in people with autism across the lifespan. Cognitive abilities in childhood seem to play a big role in cognitive function in adulthood, although many children show academic challenges even with normal to high IQ92. Even in younger children, there is great variability in the trajectories of adaptive behavior, which is a measure of functioning in individuals with autism. Some improve over time, and some decline93. Understanding the differences between people with autism whose symptoms stay stable vs. those that decline may be critically important to future diagnosis and intervention studies. These studies emphasize the importance of early intervention, a tailored individualized approach, and also academic and social support across the lifespan.
What it all means
There were a number of exciting advances in understanding autism in 2018. Researchers made progress in identifying subgroups of ASD, defining biological markers, and developing interventions. There were also studies that demonstrate that while autism is a spectrum itself, it is also part of a bigger spectrum of neurodevelopmental disorders from anxiety to ADHD to OCD. Therefore, the approaches to these other conditions may be applicable to ASD. In addition, there may be more similarities than differences in the biological features of these conditions.
Autism is distinct from other diagnoses, and despite the vast differences in symptom presentation, onset, and severity, findings call for improved access to services across all intellectual abilities and through different generations.
References:
- Davignon MN, Qian Y, Massolo M, Croen LA. Psychiatric and Medical Conditions in Transition-Aged Individuals With ASD.Pediatrics. 2018;141(Suppl 4):S335-S345.
- Septier M, Peyre H, Amsellem F, et al. Increased risk of ADHD in families with ASD. Eur Child Adolesc Psychiatry. 2018.
- Chen MH, Hsu JW, Huang KL, et al. Risk and coaggregation of major psychiatric disorders among first-degree relatives of patients with bipolar disorder: a nationwide population-based study. Psychol Med. 2018:1-8.
- Cheng CM, Chang WH, Chen MH, et al. Co-aggregation of major psychiatric disorders in individuals with first-degree relatives with schizophrenia: a nationwide population-based study. Mol Psychiatry. 2018;23(8):1756-1763.
- Karalunas SL, Hawkey E, Gustafsson H, et al. Overlapping and Distinct Cognitive Impairments in Attention-Deficit/Hyperactivity and Autism Spectrum Disorder without Intellectual Disability. J Abnorm Child Psychol. 2018;46(8):1705-1716.
- Berenguer C, Rosello B, Colomer C, Baixauli I, Miranda A. Children with autism and attention deficit hyperactivity disorder. Relationships between symptoms and executive function, theory of mind, and behavioral problems. Res Dev Disabil. 2018;83:260-269.
- Hollocks MJ, Lerh JW, Magiati I, Meiser-Stedman R, Brugha TS. Anxiety and depression in adults with autism spectrum disorder: a systematic review and meta-analysis. Psychol Med. 2018:1-14.
- Shephard E, Bedford R, Milosavljevic B, et al. Early developmental pathways to childhood symptoms of attention-deficit hyperactivity disorder, anxiety and autism spectrum disorder. J Child Psychol Psychiatry. 2018.
- Neuhaus E, Bernier RA, Tham SW, Webb SJ. Gastrointestinal and Psychiatric Symptoms Among Children and Adolescents With Autism Spectrum Disorder. Front Psychiatry. 2018;9:515.
- Kogan MD, Vladutiu CJ, Schieve LA, et al. The Prevalence of Parent-Reported Autism Spectrum Disorder Among US Children. Pediatrics.2018;142(6).
- Karpur A, Lello A, Frazier T, Dixon PJ, Shih AJ. Health Disparities among Children with Autism Spectrum Disorders: Analysis of the National Survey of Children’s Health 2016. J Autism Dev Disord. 2018.
- Candon MK, Barry CL, Marcus SC, et al. Insurance Mandates and Out-of-Pocket Spending for Children With Autism Spectrum Disorder. Pediatrics.2018.
- Gandal MJ, Haney JR, Parikshak NN, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359(6376):693-697.
- Yu Q, He Z, Zubkov D, et al. Lipidome alterations in human prefrontal cortex during development, aging, and cognitive disorders. Mol Psychiatry. 2018.
- Gandal MJ, Zhang P, Hadjimichael E, et al. Transcriptome-wide isoform-level dysregulation in ASD, schizophrenia, and bipolar disorder. Science. 2018;362(6420).
- Brainstorm C, Anttila V, Bulik-Sullivan B, et al. Analysis of shared heritability in common disorders of the brain. Science. 2018;360(6395).
- Pettersson E, Lichtenstein P, Larsson H, et al. Genetic influences on eight psychiatric disorders based on family data of 4 408 646 full and half-siblings, and genetic data of 333 748 cases and controls. Psychol Med. 2018:1-8.
- De Rubeis S, Siper PM, Durkin A, et al. Delineation of the genetic and clinical spectrum of Phelan-McDermid syndrome caused by SHANK3 point mutations. Mol Autism. 2018;9:31.
- Abbeduto L, Thurman AJ, McDuffie A, et al. ASD Comorbidity in Fragile X Syndrome: Symptom Profile and Predictors of Symptom Severity in Adolescent and Young Adult Males. J Autism Dev Disord. 2018.
- Vismara LA, McCormick CEB, Shields R, Hessl D. Extending the Parent-Delivered Early Start Denver Model to Young Children with Fragile X Syndrome. J Autism Dev Disord. 2018.
- Steinmetz AB, Stern SA, Kohtz AS, Descalzi G, Alberini CM. Insulin-Like Growth Factor II Targets the mTOR Pathway to Reverse Autism-Like Phenotypes in Mice. J Neurosci. 2018;38(4):1015-1029.
- Wegiel J, Brown WT, La Fauci G, et al. The role of reduced expression of fragile X mental retardation protein in neurons and increased expression in astrocytes in idiopathic and syndromic autism (duplications 15q11.2-q13). Autism Res. 2018;11(10):1316-1331.
- Parikshak NN, Swarup V, Belgard TG, et al. Genome-wide changes in lncRNA, splicing, and regional gene expression patterns in autism. Nature. 2016;540(7633):423-427.
- Baio J, Wiggins L, Christensen DL, et al. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years – Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1-23.
- Schendel DE, Thorsteinsson E. Cumulative Incidence of Autism Into Adulthood for Birth Cohorts in Denmark, 1980-2012. JAMA. 2018;320(17):1811-1813.
- Ozonoff S, Young GS, Brian J, et al. Diagnosis of Autism Spectrum Disorder After Age 5 in Children Evaluated Longitudinally Since Infancy. J Am Acad Child Adolesc Psychiatry. 2018;57(11):849-857 e842.
- Harrop C, Jones D, Zheng S, Nowell SW, Boyd BA, Sasson N. Sex differences in social attention in autism spectrum disorder. Autism Res. 2018;11(9):1264-1275.
- Harrop C, Jones D, Zheng S, Nowell S, Boyd BA, Sasson N. Circumscribed Interests and Attention in Autism: The Role of Biological Sex. J Autism Dev Disord. 2018;48(10):3449-3459.
- Lai MC, Lombardo MV, Chakrabarti B, et al. Neural self-representation in autistic women and association with ‘compensatory camouflaging’. Autism. 2018:1362361318807159.
- Lombardo MV, Auyeung B, Pramparo T, et al. Sex-specific impact of prenatal androgens on social brain default mode subsystems. Mol Psychiatry. 2018.
- Quartier A, Chatrousse L, Redin C, et al. Genes and Pathways Regulated by Androgens in Human Neural Cells, Potential Candidates for the Male Excess in Autism Spectrum Disorder. Biol Psychiatry. 2018;84(4):239-252.
- Cherskov A, Pohl A, Allison C, Zhang H, Payne RA, Baron-Cohen S. Polycystic ovary syndrome and autism: A test of the prenatal sex steroid theory. Transl Psychiatry. 2018;8(1):136.
- Chen G, Jin Z, Li S, et al. Early life exposure to particulate matter air pollution (PM1, PM2.5 and PM10) and autism in Shanghai, China: A case-control study. Environ Int. 2018.
- Kalkbrenner AE, Windham GC, Zheng C, et al. Air Toxics in Relation to Autism Diagnosis, Phenotype, and Severity in a U.S. Family-Based Study. Environ Health Perspect. 2018;126(3):037004.
- Mandy W, Pellicano L, St Pourcain B, Skuse D, Heron J. The development of autistic social traits across childhood and adolescence in males and females. J Child Psychol Psychiatry. 2018;59(11):1143-1151.
- La Buissonniere-Ariza V, Wood JJ, Kendall PC, et al. Presentation and Correlates of Hoarding Behaviors in Children with Autism Spectrum Disorders and Comorbid Anxiety or Obsessive-Compulsive Symptoms. J Autism Dev Disord. 2018;48(12):4167-4178.
- Pagalan L, Bickford C, Weikum W, et al. Association of Prenatal Exposure to Air Pollution With Autism Spectrum Disorder. JAMA Pediatr. 2018.
- Brown AS, Cheslack-Postava K, Rantakokko P, et al. Association of Maternal Insecticide Levels With Autism in Offspring From a National Birth Cohort. Am J Psychiatry. 2018:appiajp201817101129.
- Croen LA, Qian Y, Ashwood P, et al. Family history of immune conditions and autism spectrum and developmental disorders: Findings from the study to explore early development.Autism Res. 2018.
- Rubenstein E, Young JC, Croen LA, et al. Brief Report: Maternal Opioid Prescription from Preconception Through Pregnancy and the Odds of Autism Spectrum Disorder and Autism Features in Children. J Autism Dev Disord. 2018.
- Wimberley T, Agerbo E, Pedersen CB, et al. Otitis media, antibiotics, and risk of autism spectrum disorder. Autism Res. 2018;11(10):1432-1440.
- Curtin P, Austin C, Curtin A, et al. Dynamical features in fetal and postnatal zinc-copper metabolic cycles predict the emergence of autism spectrum disorder.Sci Adv. 2018;4(5):eaat1293.
- Schwede M, Nagpal S, Gandal MJ, et al. Strong correlation of downregulated genes related to synaptic transmission and mitochondria in post-mortem autism cerebral cortex. J Neurodev Disord. 2018;10(1):18.
- An JY, Lin K, Zhu L, et al. Genome-wide de novo risk score implicates promoter variation in autism spectrum disorder. Science.2018;362(6420).
- Woodbury-Smith M, Scherer SW. Progress in the genetics of autism spectrum disorder. Dev Med Child Neurol. 2018;60(5):445-451.
- Woodbury-Smith M, Paterson AD, O’Connor I, et al. A genome-wide linkage study of autism spectrum disorder and the broad autism phenotype in extended pedigrees. J Neurodev Disord. 2018;10(1):20.
- Brandler WM, Antaki D, Gujral M, et al. Paternally inherited cis-regulatory structural variants are associated with autism. Science.2018;360(6386):327-331.
- Williams SM, An JY, Edson J, et al. An integrative analysis of non-coding regulatory DNA variations associated with autism spectrum disorder. Mol Psychiatry. 2018.
- Werling DM, Brand H, An JY, et al. An analytical framework for whole-genome sequence association studies and its implications for autism spectrum disorder. Nat Genet. 2018;50(5):727-736.
- Willingham E. Optimism, confusion greet federal fast track for autism drug. Spectrum News; 2018.
- Berg EL, Copping NA, Rivera JK, et al. Developmental social communication deficits in the Shank3 rat model of phelan-mcdermid syndrome and autism spectrum disorder. Autism Res. 2018;11(4):587-601.
- Hulbert SW, Bey AL, Jiang YH. Environmental enrichment has minimal effects on behavior in the Shank3 complete knockout model of autism spectrum disorder. Brain Behav. 2018;8(11):e01107.
- Qin L, Ma K, Wang ZJ, et al. Social deficits in Shank3-deficient mouse models of autism are rescued by histone deacetylase (HDAC) inhibition. Nat Neurosci. 2018;21(4):564-575.
- Dalla Vecchia E, Mortimer N, Palladino VS, et al. Cross-species models of attention-deficit/hyperactivity disorder and autism spectrum disorder: lessons from CNTNAP2, ADGRL3, and PARK2.Psychiatr Genet. 2018.
- Jung H, Park H, Choi Y, et al. Sexually dimorphic behavior, neuronal activity, and gene expression in Chd8-mutant mice. Nat Neurosci.2018;21(9):1218-1228.
- Zerbi V, Ielacqua GD, Markicevic M, et al. Dysfunctional Autism Risk Genes Cause Circuit-Specific Connectivity Deficits With Distinct Developmental Trajectories. Cereb Cortex. 2018;28(7):2495-2506.
- DeRosa BA, El Hokayem J, Artimovich E, et al. Convergent Pathways in Idiopathic Autism Revealed by Time Course Transcriptomic Analysis of Patient-Derived Neurons. Sci Rep. 2018;8(1):8423.
- Williams M, Prem S, Zhou X, et al. Rapid Detection of Neurodevelopmental Phenotypes in Human Neural Precursor Cells (NPCs). J Vis Exp. 2018(133).
- Nguyen LS, Fregeac J, Bole-Feysot C, et al. Role of miR-146a in neural stem cell differentiation and neural lineage determination: relevance for neurodevelopmental disorders. Mol Autism.2018;9:38.
- Marrus N, Hall LP, Paterson SJ, et al. Language delay aggregates in toddler siblings of children with autism spectrum disorder. J Neurodev Disord. 2018;10(1):29.
- Henry L, Farmer C, Manwaring SS, Swineford L, Thurm A. Trajectories of cognitive development in toddlers with language delays. Res Dev Disabil. 2018;81:65-72.
- Bosl WJ, Tager-Flusberg H, Nelson CA. EEG Analytics for Early Detection of Autism Spectrum Disorder: A data-driven approach. Sci Rep. 2018;8(1):6828.
- Capal JK, Carosella C, Corbin E, Horn PS, Caine R, Manning-Courtney P. EEG endophenotypes in autism spectrum disorder. Epilepsy Behav. 2018;88:341-348.
- Shen MD, Nordahl CW, Li DD, et al. Extra-axial cerebrospinal fluid in high-risk and normal-risk children with autism aged 2-4 years: a case-control study. Lancet Psychiatry. 2018;5(11):895-904.
- Kim SH, Grzadzinski R, Martinez K, Lord C. Measuring treatment response in children with autism spectrum disorder: Applications of the Brief Observation of Social Communication Change to the Autism Diagnostic Observation Schedule. Autism. 2018:1362361318793253.
- Waters CF, Amerine Dickens M, Thurston SW, Lu X, Smith T. Sustainability of Early Intensive Behavioral Intervention for Children With Autism Spectrum Disorder in a Community Setting. Behav Modif. 2018:145445518786463.
- Iadarola S, Shih W, Dean M, et al. Implementing a Manualized, Classroom Transition Intervention for Students With ASD in Underresourced Schools. Behav Modif. 2018;42(1):126-147.
- Bal VH, Hendren RL, Charman T, et al. Considerations from the 2017 IMFAR Preconference on Measuring Meaningful Outcomes from School-Age to Adulthood. Autism Res. 2018;11(11):1446-1454.
- Scott M, Milbourn B, Falkmer M, et al. Factors impacting employment for people with autism spectrum disorder: A scoping review. Autism.2018:1362361318787789.
- Anderson KA, Sosnowy C, Kuo AA, Shattuck PT. Transition of Individuals With Autism to Adulthood: A Review of Qualitative Studies. Pediatrics.2018;141(Suppl 4):S318-S327.
- Eack SM, Hogarty SS, Greenwald DP, et al. Cognitive enhancement therapy for adult autism spectrum disorder: Results of an 18-month randomized clinical trial. Autism Res. 2018;11(3):519-530.
- Hatfield M, Falkmer M, Falkmer T, Ciccarelli M. Effectiveness of the BOOST-A online transition planning program for adolescents on the autism spectrum: a quasi-randomized controlled trial. Child Adolesc Psychiatry Ment Health. 2017;11:54.
- Veenstra-VanderWeele J. Recognizing the Problem of Suicidality in Autism Spectrum Disorder. J Am Acad Child Adolesc Psychiatry. 2018;57(5):302-303.
- Culpin I, Mars B, Pearson RM, et al. Autistic Traits and Suicidal Thoughts, Plans, and Self-Harm in Late Adolescence: Population-Based Cohort Study. J Am Acad Child Adolesc Psychiatry. 2018;57(5):313-320 e316.
- Cassidy SA, Bradley L, Bowen E, Wigham S, Rodgers J. Measurement properties of tools used to assess suicidality in autistic and general population adults: A systematic review. Clin Psychol Rev. 2018;62:56-70.
- Cassidy S, Bradley L, Shaw R, Baron-Cohen S. Risk markers for suicidality in autistic adults. Mol Autism. 2018;9:42.
- Horowitz LM, Thurm A, Farmer C, et al. Talking About Death or Suicide: Prevalence and Clinical Correlates in Youth with Autism Spectrum Disorder in the Psychiatric Inpatient Setting. J Autism Dev Disord. 2018;48(11):3702-3710.
- Tromans S, Chester V, Kiani R, Alexander R, Brugha T. The Prevalence of Autism Spectrum Disorders in Adult Psychiatric Inpatients: A Systematic Review. Clin Pract Epidemiol Ment Health. 2018;14:177-187.
- Siegel M. The Severe End of the Spectrum: Insights and Opportunities from the Autism Inpatient Collection (AIC). J Autism Dev Disord. 2018;48(11):3641-3646.
- Stedman A, Taylor B, Erard M, Peura C, Siegel M. Are Children Severely Affected by Autism Spectrum Disorder Underrepresented in Treatment Studies? An Analysis of the Literature. J Autism Dev Disord. 2018.
- Handen BL, Mazefsky CA, Gabriels RL, et al. Risk Factors for Self-injurious Behavior in an Inpatient Psychiatric Sample of Children with Autism Spectrum Disorder: A Naturalistic Observation Study. J Autism Dev Disord. 2018;48(11):3678-3688.
- Sannar EM, Palka T, Beresford C, et al. Sleep Problems and Their Relationship to Maladaptive Behavior Severity in Psychiatrically Hospitalized Children with Autism Spectrum Disorder (ASD). J Autism Dev Disord. 2018;48(11):3720-3726.
- Szaflarski JP, Bebin EM, Cutter G, et al. Cannabidiol improves frequency and severity of seizures and reduces adverse events in an open-label add-on prospective study. Epilepsy Behav. 2018.
- Devinsky O, Verducci C, Thiele EA, et al. Open-label use of highly purified CBD (Epidiolex(R)) in patients with CDKL5 deficiency disorder and Aicardi, Dup15q, and Doose syndromes. Epilepsy Behav. 2018;86:131-137.
- Aran A, Cassuto H, Lubotzky A, Wattad N, Hazan E. Brief Report: Cannabidiol-Rich Cannabis in Children with Autism Spectrum Disorder and Severe Behavioral Problems-A Retrospective Feasibility Study. J Autism Dev Disord. 2018.
- Hadjikhani N, Asberg Johnels J, Lassalle A, et al. Bumetanide for autism: more eye contact, less amygdala activation. Sci Rep. 2018;8(1):3602.
- Avino TA, Barger N, Vargas MV, et al. Neuron numbers increase in the human amygdala from birth to adulthood, but not in autism. Proc Natl Acad Sci U S A. 2018;115(14):3710-3715.
- Moazen-Zadeh E, Shirzad F, Karkhaneh-Yousefi MA, Khezri R, Mohammadi MR, Akhondzadeh S. Simvastatin as an Adjunctive Therapy to Risperidone in Treatment of Autism: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J Child Adolesc Psychopharmacol. 2018;28(1):82-89.
- Stivaros S, Garg S, Tziraki M, et al. Randomised controlled trial of simvastatin treatment for autism in young children with neurofibromatosis type 1 (SANTA). Mol Autism. 2018;9:12.
- Morgan L, Hooker JL, Sparapani N, Reinhardt VP, Schatschneider C, Wetherby AM. Cluster randomized trial of the classroom SCERTS intervention for elementary students with autism spectrum disorder. J Consult Clin Psychol. 2018;86(7):631-644.
- Bal VH, Kim SH, Fok M, Lord C. Autism spectrum disorder symptoms from ages 2 to 19 years: Implications for diagnosing adolescents and young adults. Autism Res. 2018.
- Kim SH, Bal VH, Lord C. Longitudinal follow-up of academic achievement in children with autism from age 2 to 18. J Child Psychol Psychiatry. 2018;59(3):258-267.
- Sacrey LR, Zwaigenbaum L, Bryson S, et al. Developmental trajectories of adaptive behavior in autism spectrum disorder: a high-risk sibling cohort. J Child Psychol Psychiatry. 2018.


