Joseph Kraft is a medical doctor who measured over 14,000 oral glucose tolerance tests in his lifetime. This is a standard test to measure the blood glucose response to a standardized amount of glucose over 2 hours. The difference is that he measured over 5 hours and included blood insulin levels. A summary of his work is here and Prof Grant also reviews it nicely here. Ivor Cummins, The Fat Emperor, has also reviewed it nicely here.
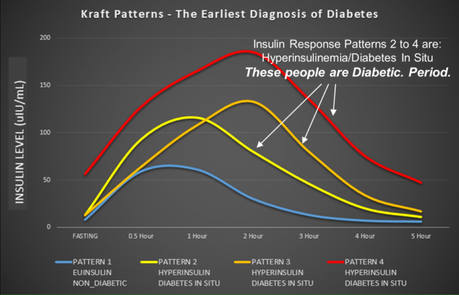
But people with normal OGTT may still have an abnormal insulin response. Those people who respond with excessive secretion of insulin to 75g of glucose are at very high risk of eventually developing T2D as well. So the insulin response is even earlier, which means you can diagnose 'diabetes in situ', which means incipient diabetes.
Incipient diabetes
Let's think about this for a second. This makes a lot of sense. If you simply wait until blood glucose is elevated, then you have T2D, no question. But if you have normal blood sugars, then you may still be at risk of diabetes (pre-diabetes). So, we give a big load of glucose and see if the body is able to handle it. If this is negative as well, this does not yet mean everything is normal.
If the body responds by very high secretion of insulin, this will force the blood glucose into the cell and keep the blood glucose normal. But this is not normal. It's like the trained athlete who can easily run 10K in 1 hour and the untrained athlete who must dig deep and use all his effort to do so. Those people who need to produce prodigious amounts of insulin to force the glucose back to normal are at high risk. This makes perfect physiologic sense. But there's a much deeper implication to this:
HYPERINSULINEMIA PRECEDES HYPERGLYCEMIA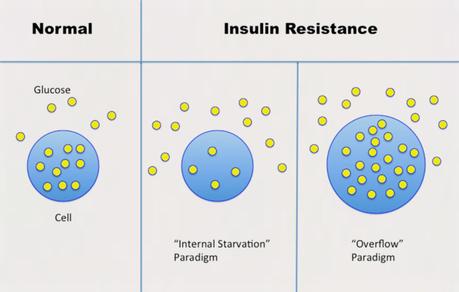
In the standard 'internal starvation' model, some unknown thing (inflammation, oxidative stress etc.) causes IR, which blocks glucose from entering the cell. It looks like this:
IR -> hyperglycemia -> hyperinsulinemiaThis is completely incorrect because the hyperglycaemia PRECEDES the hyperinsulinemia. According to the this theory, we still need to find the mysterious boogeyman that causes IR. There are those, for example that claim dietary fat causes IR, others say vegetable oil, inflammation, oxidative stress, genes etc. But it simply is not correct because the high insulin comes first. So therefore the high blood glucose cannot CAUSE the high insulin.
But according to the 'overflow' model, things look like this.
Too much sugar -> hyperinsulinemia -> fatty liver and IR -> hyperglycaemiaThe implication of Krafts pioneering work is this - The 'Internal Starvation' paradigm is completely backwards. Think about this. If we think that T2D is the result of internal starvation, would we expect internal starvation to look like it does? (Large waist, obesity, fatty liver) What part of that looks like internal starvation of cells? This means that the high insulin causes the high blood glucose (symptom of the disease). Therefore, the proper treatment of T2D is to LOWER INSULIN. How? Drugs don't do this, in general. It would require dietary changes - LCHF and Intermittent Fasting.
Overflow paradigm
Picture a subway train in the middle of rush hour. Each train stops at a station and upon getting the 'all clear' signal, opens its doors. Some passengers leave but most go into the train on their way to or from work. All the passengers go into the train without problems and the platform is empty as the train pulls out.
A cell works in an analogous method. When insulin gives the proper signal, the gates open and glucose enters the cell in an orderly fashion without much difficulty. The cell is like the subway train, and the passengers are like the glucose molecules.
When the cell is insulin resistant, insulin signals the cell to open the doors, but no glucose enters. Glucose accumulates in the blood, unable to get inside the cell. In our train analogy, the train pulls into the station, receives the signal to open the doors, but no passengers enter the train. As the train pulls out, many passengers are left on the platform, unable to enter the train.
Why does this happen?
There are several possibilities. Under the 'lock and key' paradigm, the interaction of insulin with its receptor fails to fully open the gate. This leaves glucose outside in the blood while the cell experiences internal starvation. In the train analogy, the conductor's signal fails to open the subway doors fully so passengers are unable to pass through. They are left outside on the platform, while the inside of the train is relatively empty.
But that's not the sole possibility. What happens if the train is not empty, but already jam-packed full of passengers from the previous stop? Passengers are crowded and waiting on the platform. The conductor gives the signal to open the door, but passengers cannot enter. The train is already full, so passengers are left waiting on the platform. Not because the door failed to open, but because the train is already overflowing. From the outside, it appears that passengers are not able to enter the train when the door opens.
The same situation can happen in the cell, particularly the liver. If the cell is already jam-packed full of glucose, then more cannot enter despite the fact that insulin has opened the gate. From the outside, we can only say that the cell is 'resistant' to insulin's urging to move glucose inside. But this is not a gummed up 'lock and key' mechanism. This is an overflow phenomenon.
So how do we go about this?
In the train analogy, what can you do to pack more people into the train? One solution is simply to hire "subway pushers" to shove people into the trains. This was implemented in New York City in the 1920's. While these practices died out in North America, they still exist in Japan, where they are called "passenger arrangement staff".

Let's think about the liver cell. At birth the liver is empty of glucose. When we eat, glucose enters the liver cell. When we don't eat, or fast, glucose leaves. With persistently high insulin levels, glucose keeps entering the liver cell. Over decades, glucose slowly fills the cell until it is now overflowing like the congested subway train. When the gate opens for glucose to enter, it is unable to do so. The cell is now insulin resistant. Hyperinsulinemia creates the insulin resistance.
To compensate, insulin levels increase and like the Japanese Subway Pushers, tries to push more glucose into the cell by force. The insulin resistance creates hyperinsulinemia, the very thing that created it. This works, but only for a short while, because eventually there is no more room for the glucose. The vicious cycle goes round and round, worsening with each iteration.
The cell is not in a state of 'internal starvation', but rather, it is overflowing with glucose. As it spills out of the cell, blood glucose levels increase. Insulin's action on glucose is being resisted. But what happens to insulin's other major job to increase new fat production or DNL? If the cell is truly resistant to insulin, DNL should decrease.
Is there a better way?
But the cell is overfilled with glucose, not empty, so there is no reduction of DNL. Instead, the cell is producing as much new fat as possible to relieve the internal congestion. The action of insulin to increase DNL is not being resisted, but enhanced. This paradigm perfectly explains the central paradox.
On the one hand, the cell is resistant to insulin's effect on glucose. On the other, insulin's effect on DNL is enhanced. This happens in the liver cell, with the same level of insulin and the same insulin receptors. The paradox has been resolved by understanding this new paradigm of insulin resistance. The cell is not in a state of 'internal starvation', but rather a 'glucose overload' one.
As the liver ramps up DNL to deal with its internal congestion, more new fat is created than can be exported. Fat backs up in the liver, an organ not designed for fat storage. This disease of fatty liver is intimately related to the overflow problem of insulin resistance.
Understanding this new paradigm is critical. According to the old 'lock and key' paradigm, the treatment of T2D involved hiring more subway pushers to shove even more passengers into the crowded train. This is analogous to giving more insulin to patients, even though we already know that insulin is too high. If we understand the 'overflow' paradigm, we see that the logical treatment of type 2 diabetes is to empty out the train. How? LCHF diets, intermittent fasting. In other words, type 2 diabetes is essentially just a disease of too much sugar in the body. The only logical treatments, therefore are to:
- Stop putting sugar in (LCHF)
- Burn the sugar off (Intermittent Fasting)
That's all you need to know to reverse type 2 diabetes.
-
Jason Fung

