Dr. Alfred Frohlich from the University of Vienna first began to unravel the neuro-hormonal basis of obesity in 1890. He described a young boy with the sudden onset of obesity who was eventually diagnosed with a lesion in the hypothalamus area of the brain. It would be later confirmed that hypothalamic damage resulted in intractable weight gain in humans, establishing this region as a key regulator of energy balance.
In rats and other animals, hypothalamic injury could experimentally produce insatiable appetites and induce obesity. But, researchers quickly noticed something else, too. All these obese animals shared characteristic liver damage, which was occasionally severe enough progress to complete destruction. Looking back at hereditarily obese strains of mice, they noted the same liver changes. Strange, they thought. What does the liver have to do with obesity?
Dr. Samuel Zelman first made the connection between liver disease and obesity in 1952. He observed fatty liver disease in a hospital aide who drank in excess of twenty bottles of Coca-Cola daily. This was already a well-known complication of alcoholism, but this patient did not drink alcohol. That obesity could cause similar liver damage by itself was completely unknown at that time. Zelman, aware of the animal data, spent the next few years tracking down twenty other obese, non-alcoholic patients with evidence of liver disease. One oddity he noted was that they unanimously preferred carbohydrate rich diets.
Fatty liver in patients that are not alcoholics
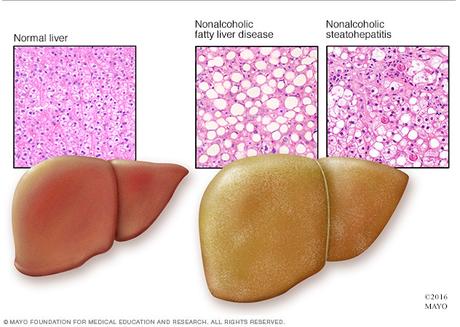
This discovery, at the very minimum, saved patients from their doctor's repeated accusations that they were lying about their alcohol intake. Dr. Ludwig wrote that it spared doctors "the embarrassment (or worse) that may result from the ensuing verbal exchanges." Some things in life never change. Today, when patients have difficulty losing weight on an 'Eat Less, Move More' diet, doctors accuse patients about cheating, rather than accept the bitter fact that this diet simply does not work. This age-old game is called "Blame the Victim".
With the new recognition of NAFLD, research confirmed the extraordinarily close association between obesity, insulin resistance and fatty liver. Obese individuals have five to fifteen times the rate of fatty liver. Up to 85% of type 2 diabetics have fatty liver. Even without the diabetes, those with insulin resistance alone have higher levels of liver fat. These three diseases clearly clustered together. Where you found one, you almost invariable found the others.
Hepatic steatosis - the deposition of fat in the liver where it should not be, is consistently one of the most important markers of insulin resistance. The degree of insulin resistance is directly related to the amount of fat in the liver. Rising alanine transaminase levels, a blood marker of liver damage, in obese children are directly linked to insulin resistance and the development of type 2 diabetes. Even independent of obesity, severity of fatty liver correlates to pre-diabetes, insulin resistance and impairment of beta cell function.
This is a truly frightening epidemic. In the space of a single generation, this disease went from being completely unknown, with even a name, to the commonest cause of liver disease in the North America. From virtual unknown to world heavyweight champion of the world, this is the Rocky Balbao of liver diseases.
Fatty liver - the core issue
The liver lies at the nexus of food energy storage and production. After absorption through the intestines, nutrients are delivered directly through the portal circulation to the liver. Since body fat is essentially a method of food energy storage, it is little wonder that diseases of fat storage involve the liver intimately.
Insulin pushes glucose into the liver cell, gradually filling it up. The liver turns on DNL to convert this excess glucose to fat, the storage form of food energy. Too much glucose, and too much insulin, over too long a period of time leads eventually to fatty liver.
Insulin resistance is an overflow phenomenon, where glucose is unable to enter the cell that is already overfilled. Fatty liver, a manifestation of these overfilled cells, creates insulin resistance. The cycle proceeds as follows:
- Hyperinsulinemia causes fatty liver.
- Fatty liver causes insulin resistance.
- Insulin resistance leads to compensatory hyperinsulinemia.
Hyperinsulinemia causes insulin resistance, and is the initial trigger for this vicious cycle.
Fatty liver leads to type 2 diabetes
Fatty liver is clearly associated at all stages from mere insulin resistance to pre-diabetes to full blown diabetes, even independent of overall obesity. This relationship holds in all ethnicities, whether Asian, Caucasian or African-American.
The crucial piece necessary for development of insulin resistance is not overall obesity, but fat contained within the liver, where there should not be any. This is the reason that there are underweight patients, as defined by the Body Mass Index, who still suffer from type 2 diabetes. These patients are often termed "skinny diabetics" or TOFI (Thin on the Outside, Fat on the Inside). Overall weight matters less than the fat carried around the midsection and the liver. This central obesity, rather than generalized obesity is characteristic of the metabolic syndrome and type 2 diabetes.
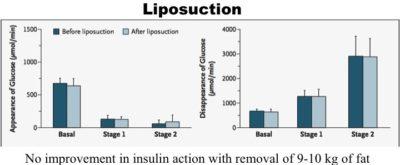
The surgical removal of large amounts of subcutaneous fat reduces body weight, BMI, waist circumference and the hormone leptin decreases. However, metabolic parameters are not improved even with the removal of ten kilograms (22 pounds) of subcutaneous fat. There are no measurable benefits in blood glucose, insulin resistance, inflammatory markers or lipid profiles. This lack of benefit occurs despite similar levels of fat loss compared to most dietary weight loss programs.
By contrast, weight loss through dietary intervention often result in marked improvement in all metabolic parameters. Conventional weight loss methods reduce subcutaneous fat, visceral fat, and intra hepatic fat whereas liposuction only removes subcutaneous fat.
Visceral fat is a far superior predictor of diabetes, dyslipidemia and heart disease compared to overall obesity. But a difference still exists between fat within the organ and fat around the organs (omental fat). Direct surgical removal of omental fat also carries no metabolic benefits.
Fatty liver and insulin resistance
Fat contained within the organs in other than the liver also plays a leading role in disease. Little fat is normally contained directly within organs, and this abnormality causes most of the complications of obesity (18). This includes the fat contained within the liver, but as we shall see later, also the fat contained with the skeletal muscles and pancreas.
Fatty liver precedes the diabetes diagnosis often by ten years or more. The emergence of the metabolic syndrome follows a consistent sequence. Weight gain, even as little as 2 kilograms (4.4 pounds) is the first detectable abnormality, followed by low HDL cholesterol levels. High blood pressure, fatty liver, and high triglycerides emerge next, at roughly the same time. The very last symptom to appear was the high blood sugars. This is a late finding in metabolic syndrome.
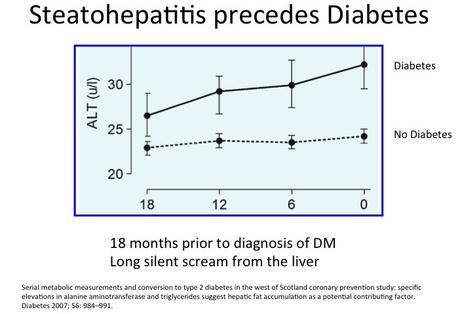
The West of Scotland study confirmed that fatty liver and elevated triglycerides precedes the diagnosis of type 2 diabetes by at least 18 months. The triglyceride level increased more than 6 months before the diagnosis. This is strong evidence that accumulation of liver fat is crucial to the development of insulin resistance, but also may act as a trigger for the development of type 2 diabetes.
In type 2 diabetic patients, there is a close correlation between the amount of liver fat and the insulin dose required reflecting greater insulin resistance. In short, the fattier the liver, the higher the insulin resistance.
By contrast, in type 1 diabetes, insulin levels are extremely low, and liver fat is lower than normal. This is strong evidence that insulin levels are a key causal factor in developing fatty liver. Insulin drives fat production in the liver and low levels of insulin lead to less fat in the liver.
-
Jason Fung
How to cure fatty liver
Several studies show that a low-carb diet reduces fatty liver, probably by lowering insulin:
Do you want to try a low-carb diet? Check out our freelow-carb guide or sign up for our free two-week low-carb challenge.

