I was diagnosed with Superficial siderosis in 2015, but it was late 2017 before I finally found a neurologist who was willing to listen and help me in any way he could. He agreed deferiprone (Ferriprox) was the only treatment available, and although not tolerated by all, I should try. It took a few months for permission due to the fact it’s an off-label medication. It is approved for treating Thalassemia and requires permission In Australia (and other countries) to prescribe it for other conditions. Deferiprone is an iron chelator; it removes iron and other metals from your body. It is hoped that when used in conjunction with a low iron diet, it would cross the brain barrier and remove the toxic free iron (siderosis) from my brain.
I had ‘benchmark’ tests completed in February 2018. These included blood tests and a baseline brain MRI to see where we were starting from to compare down the track. In addition, I had hearing tests that showed my hearing was not yet affected by the siderosis. I also had a lumbar puncture to check for an active bleed which was clear.
As I was having many problems with my eyes at the time – not vision problems, but optic neuritis type eye pain and light sensitivity, my neurologist, arranged a visual evoked potentials test. It is a test widely used in the diagnosis of MS. It involves watching a moving checkerboard screen with alternate eyes covered and pressing a button when you see a red dot. There are electrodes attached to your scalp, and it measures how fast the signal travels from your eyes to your brain. It detects damage to the optic nerve. My results for this test were extremely poor, and in fact, my score was so low for one eye it didn’t make it onto the charts. The other barely did. My MRI results had indicated I had hemosiderin deposits on my optic nerves, so these results were consistent with the findings.
I began on deferiprone in mid-February by taking 4 tablets a day, 2 in the morning and 2 at night, 7 days a week. In the middle of the day, I needed to eat iron-rich foods, but not within two hours on either side of the tablets, leaving a small window for eating. I also needed weekly blood tests to check that my blood iron levels weren’t getting too low, which can be dangerous. So after approximately 9 months on this regime, I reduced the tablets to 5 days a week as my blood tests showed I was hovering on the border of anaemia.
In August of 2018, I resat the visual evoked potentials test. This time, the results improved so much that my results showed that I was within the normal range in both eyes. This was my earliest indication that the deferiprone appeared to be working on the optic nerves, and my neurologist was very excited and pleased with the results. I had another MRI on the 14th of August, and the results showed that my superficial siderosis in the brain was stable and unchanged.
In April 2019, another Mri was done to check progression after being on deferiprone for a year. There was no improvement, but it hadn’t gotten worse either. I still had weekly blood tests. I was also having regular hearing tests, which continued to show that my hearing was still unaffected. I continued seeing my neurologist every four or so months, with new MRIs’ being done every six months. My blood tests continued every week. A couple of times, I took a break from the medication for a few weeks as my iron levels were low.
January 2020, two years after starting chelation, another MRI was done. This one read-” In comparison with the previous MRI, the degree of susceptibility artefact in the cerebellar folia has marginally improved, suggesting treatment response.” This was my first ‘in writing’ proof that the treatment was working.
2020 was the year of covid-19 restrictions, my blood tests were changed to fortnightly, so I didn’t have to attend the medical clinic so often. So I stopped the medication a couple of times for a few weeks but otherwise continued the 4 tablets 5 days a week.
February 2021 was my most recent MRI. My neurologist rang me yesterday to discuss this one, and he’s over the moon. This one read-
“The degree of superficial siderosis in the cerebellum has not appreciably changed compared with recent study of 2020 but has significantly improved compared with the prior MRI study of 2018.“
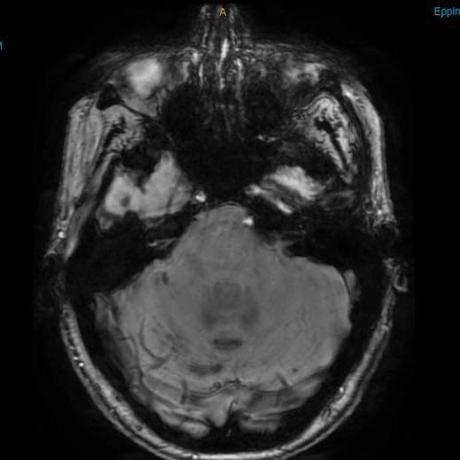
He sent me these photos of my brain. The photo on the left is the most recent; the right one is 2018. The black lines on the one on the right are the siderosis. Although the photos are slightly angles, the difference is amazing. When I asked my neurologist for measurement of improvement, he said that although it’s so hard to quantify as the MRI give measurements, he thought it was perhaps a 50% improvement.
- 2018
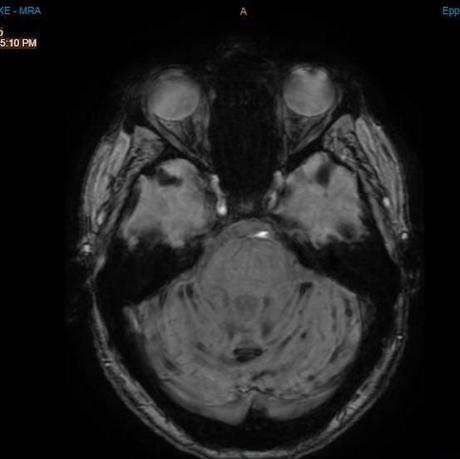
- 2021
So, it’s really been 3 years of chelation to see this improvement. Like my neuro said to me yesterday, photos like this make the anaemia and the fatigue and blood tests all worthwhile. It gives hope, excitement, and belief that this treatment can and does work. It doesn’t happen overnight, and it is frustrating and feels like Groundhog Day when you’re taking the tablets and watching the diet and not seeing any improvement. It’s an emotional rollercoaster at times, but so worth it when you see the results like this.
Every person may not well tolerate deferiprone. Therefore, using this medication for hemosiderin removal in superficial siderosis is classified as off-label use. Please follow the advice of your doctor concerning chelation therapy.
- Deferiprone can cause agranulocytosis that can lead to serious infections and death. Neutropenia may precede the development of agranulocytosis.
- Measure the absolute neutrophil count (ANC) before starting chelation therapy and monitor the ANC weekly on therapy. Interrupt chelation therapy if neutropenia develops.
- Interrupt your deferiprone use if you develop any infection or illness and monitor the ANC more frequently.
- All patients taking deferiprone should report immediately any symptoms indicative of infection to their physician.

