Yesterday Phyrangula linked to my article on the problems of trying to squeeze the ice age into a young earth creationist chronology. This earned me 10 times more page views than normal (I do hope some of you new people stick around) and resulted in a couple of questions about how isotopes can tell us about the prehistoric climate; a topic I’d glossed over in the original point. Remember, if you have questions of your own about human evolution feel free to tweet, facebook or email me (or you could leave a comment, but that’s so old fashioned).
Anyhoo, the question. Steve L asked
How do we know that isotopic signatures in corals, etc, reflect climate? Was it *because* these correlated with interglacials? If so, doesn’t that make it more difficult to use the correlation to claim that multiple lines of truly independent evidence indicates changes in climate?
Stable isotopes (compared to radioactive ones) are lovely little things that can provide us with a wealth of data on the past, from what the environment was to what individuals ate. An isotope is a variation of element that has the same protons and electrons, but a different number of neutrons. As such it behaves exactly the same, except isotopes with more neutrons are slightly heavier.
For marine isotopes (the kind used to infer palaeoclimate) this means that the lighter isotopes will rise to the surface. As such, when water evaporates from the ocean it is mostly made up of lighter isotopes; altering the ratio of heavy/light isotopes.
If this evaporated water never returns to the ocean because it freezes in glaciers, then the ratio will be permanently altered (at least until the glaciers melt). This has been confirmed by laboratory experiments and by looking at polar ice; which has a higher ratio of lighter isotopes.
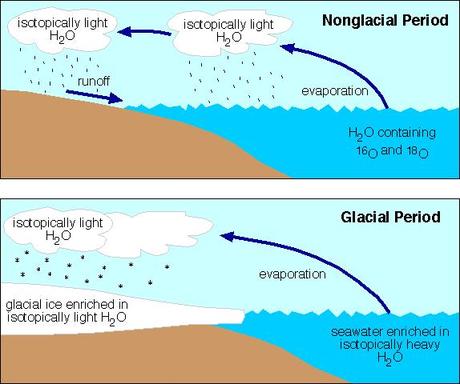
How the whole thing works
This alters the isotope ratios marine animals are exposed to, in turn altering the isotope ratios in their bodies (in much the same way that people who eat a lot of maize, which is high in certain isotopes of carbon, have a different ratio of carbon isotopes to people who don’t eat so much maize). Their bodies thus provide a snapshot of the isotope ratio of the oceans during their lifetime.
When these little organisms go to sleep for the last time, they fall to the bottom of the ocean where they form layers on the sea bed. Then it’s just a case of drilling into this grizzly piles of corpses to extract a record of marine isotopes ratios over time. Periods where there is a higher ratio of heavy isotopes, we know, correlate to periods where there were lots of glaciers on the planet (i.e. an ice age).
And that’s how science is done: by profiting from the death of billions and billions of tiny animals.

