Trusting Science
It’s natural to be reluctant to roll up your sleeve for something as new as the coronavirus vaccine. For those who also face the dual concern of balancing a rare disorder’s symptoms against contracting a highly contagious virus, anxiety levels are running high. From online discussions, it is apparent our Superficial Siderosis community has adopted a wait and see attitude.
We are all being bombarded by a lot of information right now. Some information is correct, some facts are based on just enough truth to sound reasonable, and some information is just fantasy. I can only pass along with certainty what I know to be true. I trust Dr Levy’s judgment. I trust Gary’s neurologist. You may read Dr Levy’s statement on the COVID-19 vaccine here.*
*Dr Levy’s opinion statement has had over 925 views, including SS patients, caregivers, and public internet searches.
A well used saying in the healthcare community is public health moves at the speed of trust. It makes sense, as more people in your known circles are vaccinated without consequences, the more willing you are to let go of your apprehensions. I have not heard any reports of other Superficial Siderosis patients who have already been given the vaccine. Still, I can report Gary (Shreveport LA VA Hospital) and Dr. Levy (Mass General Hospital, Boston) have been given their first dose of the Moderna COVID-19 mRNA vaccine.
Somewhere between 60 and 90 percent of adults and children must be vaccinated or have antibodies resulting from infection in order to arrive at the safe harbor known as herd immunity, where the whole community is protected.
Scientific American
Today is the fourth day past Gary’s injection. We were chatting with the nurse who was observing everyone after their shot. She said her father had experienced no reaction past a slight soreness at the injection site. Our nurse was scheduled to be given her second booster in a few days. She said she woke on her third day with an extremly sore arm that hurt to move. Two Tylenols quickly took care of the stiffness and she went on with nothing further. Other than a very slight tenderness the first day Gary has not even noticed his injection site.
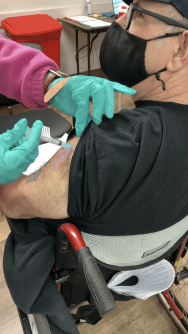
Overcoming Your Fear
The reports of allergic reactions have raised questions. A common concern is I am allergic to penicillin. Will I have a bad reaction? Gary is allergic to penicillin, so his nurse extended the post-injection observation time from 15 minutes to 30 minutes. He did not react. A severe allergic reaction would usually occur within a few minutes to one hour after getting your dose.
The FDA guidance states a person should not take the vaccine if you have experienced a past allergic reaction to any of the ingredients1 or had a reaction after your first dose. Before being vaccinated, existing allergies will be discussed during a pre-immunization interview. I am not a physician, but common sense would suggest that you should have your vaccination at a healthcare facility instead of a retail pharmacy if you have either allergy or underlying health concerns.
Pfizer/Moderna and AstraZeneca vaccines’ difference is that Pfizer and Moderna use mRNA as their platform. The Oxford/AstraZeneca uses DNA delivered to the cells by using a weakened version of a common cold virus. None of the ingredients in these vaccines can cause COVID-19.
Things To Discuss
During your pre-immunization interview or questionnaire, inform your vaccination provider about all of your medical conditions in addition to:
• any allergies you may have
• currently have a fever
• have a bleeding disorder or are on a blood thinner
• are immunocompromised or are on a medicine that affects your immune system
• are pregnant or plan to become pregnant
• are breastfeeding
• have received another COVID-19 vaccine
Known Side Effects
Side effects that have been reported with COVID-19 Vaccines include:
• injection site pain
• tiredness
• headache
• muscle pain
• chills
• joint pain
• fever
• injection site swelling
• injection site redness
• nausea
• feeling unwell
• swollen lymph nodes (lymphadenopathy)
V-Safe
V-Safe is a smartphone-based tool using text messaging and web surveys to check in with people who have recently been vaccinated to identify potential side effects after their COVID-19 vaccination. V-Safe asks questions that help the CDC monitor the safety of U.S. COVID-19 vaccinations. V-safe will also provide your second-dose reminder if needed and live telephone follow-up by a CDC representative if participants report a significant health impact following COVID-19 vaccination. Use of the V-Safe phone app is voluntary, and declining to participate will not impact your access to the vaccine.
For more information on how to sign up, visit:
www.cdc.gov/vsafe
Superficial Siderosis Survey
A patient COVID-19 survey will become available on the patient registry in the next few weeks. All surveys will be submitted to Dr. Levy so he may begin to track how our community reacts to the vaccine either neurologically or general reaction. All registered Superficial Siderosis patients will be notified when it becomes available. It will be open for answers over the course of 2021 so you may complete the survey after your second dose.
1The Pfizer-BioNTech COVID-19 vaccine ingredients include messenger ribonucleic acid (mRNA), lipids ((4-hydroxybutyl)azanediyl) bis (hexane-6,1-diyl) bis (2-hexyldecanoate), 2 [(polyethylene glycol)-2000]-N, N-ditetradecylacetamide, 1,2-Distearoyl-sn-glycero-3-phosphocholine, and cholesterol), potassium chloride, monobasic potassiumphosphate, sodium chloride, dibasic sodium phosphate dihydrate, and sucrose.
The Moderna COVID-19 Vaccine contains messenger ribonucleic acid (mRNA), lipids (SM-102, polyethylene glycol [PEG] 2000 dimyristoyl glycerol [DMG], cholesterol, and 1, 2-distearoyl-sn-glycero-3-phosphocholine [DSPC]), tromethamine, tromethamine hydrochloride, acetic acid, sodium acetate, and sucrose.
The Oxford/AstraZeneca COVID-19 vaccine contains the weakened adenovirus encoding the SARS CoV 2 Spike glycoprotein, L-histidine, L-histidine hydrochloride monohydrate, magnesium chloride hexahydrate, polysorbate 80, ethanol, sucrose, sodium chloride, disodium edetate dihydrate, water.

