 Linus Pauling, 1931.
Linus Pauling, 1931.[Ed Note: In 2009, we dipped our toes into an unusual Pauling controversy involving the theory of resonance and Soviet scientific dogma. Today we begin a much more detailed look at the “Soviet Resonance Controversy,” beginning with a detailed discussion of the scientific work that resided at the heart of the matter. This is Part 1 of 7.]
Linus Pauling’s resonance theory helped to unify the classical roots of organic chemistry with the new field of quantum physics. In so doing, the theory provided a hugely important framework for understanding observed atomic behaviors that did not correlate with then-known mathematical explanations or models of the atom.
The theory would also help to usher in an onslaught of new approaches to organic chemistry and the nature of the chemical bond, lifting, in Pauling’s words, “the veil of mystery which had shrouded the bond during the decades since its existence was first assumed.” It was, in short, one of the most adaptive and applicable postulates ever put forth by Pauling.
But the theory of resonance was not immune to controversy. Specifically, it was initially not widely accepted within the scientific community in the United States and, in a very different way, abroad in the Soviet Union. The disputes surrounding the theory were ultimately short-lived though, and Pauling’s ideas on resonance continue to inform today’s understanding of molecular architecture.
 August Kekulé
August KekuléPauling’s ideas on resonance were grounded in the work of several other scientists but most notably August Kekulé and Werner Heisenberg, both of whom were also interested in the structure of molecules.
Kekulé (1829-1896), a German chemist, notably devised a proposed structure for benzene, an aromatic hydrocarbon of interest to many. Kekulé’s model put forth a structure consisting of six carbon atoms forming a ring, with hydrogen atoms attached externally to each carbon. Though intriguing, this basic structure did not explain where, on the interior carbon ring, double bonds were located. Partly because of this, Pauling would later lament that, “the Kekulé structure for benzene is unsatisfactory.”
Shortly after Kekulé published his basic benzene structure, multiple isomers – or alternative structures – of the same compound were predicted and even isolated by Kekulé. But even these breakthroughs were not enough to explain the “correct” model of benzene. Recognizing as much, in 1872 Kekulé posited that, in actuality, benzene “oscillates” between the various isomers, and that all isomers may in fact be regarded as “correct.”
This notion of oscillation between isomers was hugely important, but despite its utility Kekulé never succeeded in accurately predicting the “true” structure of benzene. The solution to the benzene puzzle would lie in waiting for nearly sixty more years and would rely heavily upon Pauling’s resonance breakthrough.
Despite its shortcomings in accurately predicting a structure for benzene, Kekulé’s oscillation theory served well in disrupting traditionally held beliefs regarding the number of valence electrons that must be present in aromatic compounds. This, in turn, helped to usher in new theories about the chemical structure of aromatic compounds more generally.
By the 1920s, a community of American, British and German chemists had developed a set of theories related to aromatic compounds that built on Kekulé’s ideas. The group’s basic hypothesis was that, instead of molecules oscillating between various isomers, perhaps all isomers actually existed simultaneously. This idea of simultaneous existence piqued Pauling’s interest because it seemed related to work that he was doing with quantum mechanics — specifically, ideas related to quantum resonance that had been introduced by Werner Heisenberg in 1926.
 Werner Heisenberg
Werner HeisenbergHeisenberg (1901-1976), a contemporary of Pauling’s, was working to understand the wave mechanics of subatomic particles. As part of this work, he theorized that, on the subatomic level, molecules exist in quantum states – meaning discrete states – and that the actual wave function of a given molecule can be described as the sum of its various quantum states. Heisenberg coined the term resonance to refer to this process — e.g., the summation of various quantum states to comprise a molecule’s wave function.
Pauling was intimately familiar with Heisenberg’s theory of quantum resonance as well as the hypotheses proposed by the British, American, and German contingent. Thus equipped, he began to construct a theory of his own that would prove crucial to building a “truer” understanding of molecular architecture and chemical bonding.
Pauling built and circulated his resonance theory in a series of papers that were published from 1931 to 1933. In them, he reasserted the ideas stated above, before emphasizing that
the actual normal state of such a molecule does not correspond to any one of the alternative reasonable structures, but rather to a combination of them, their individual contributions being determined by their nature and stability.
In other words, the individual isomers of a given molecule should not be viewed as existing in a state of rapid switching from one to another. Instead, a hybrid of every isomer is, in fact, the “true” form of the molecule.
The distinction that Pauling drew between rapidly switching isomers – which was known as tautomerism – and isomer hybrids was conceptually difficult for many scientists to grasp, but Pauling was able to cite experimental evidence in support of his theory. Namely, Pauling had found that resonating molecules existed at a much lower energy state than tautomerism would predict. Pauling believed that these lower energy states resulted in more stable molecules, an effect that lent support to the viability of resonance – as opposed to tautomerism – as an operating theory.
The experimental data continued to be important to Pauling as he pushed his theory forward. Some had argued that there was no real difference between resonance and tautomerism, because the classical understanding of tautomerization portrayed isomers as switching so rapidly as to be in a virtual hybrid state of their own accord. But the data showed that Pauling was describing something different and that, to use Pauling’s words, “it is easy to distinguish between the two.”
In a 1946 speech delivered to a private industry group, Pauling restated the basics of his theory using language that is useful for summarizing here. For a hypothetical molecule known to have two isomers, “neither the first structure nor the second structure represents the system. Instead, the molecule is ‘a combination’ of the two structures.” And importantly, when scientists
can write two structures, neither one actually represents the state of the molecule but both of them together represent the state of the molecule. The molecule is more stable actually than it would be if it had any of the structures that you can assign to it.
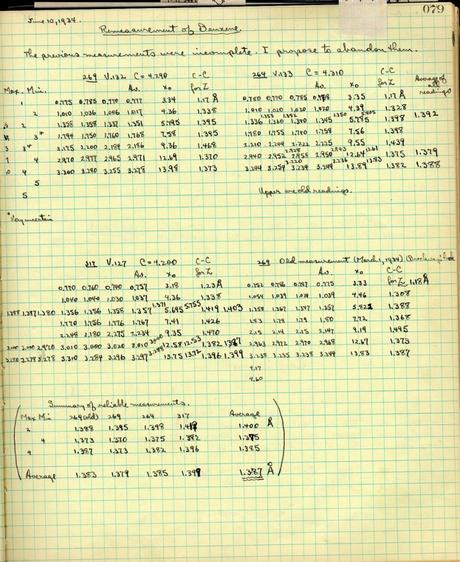 Benzene calculations in Pauling’s research notebook from June 1934
Benzene calculations in Pauling’s research notebook from June 1934Though he faced early resistance, Pauling was eventually able to persuade most of his colleagues to align with his thinking on the theory of resonance, and he did so in part by using the theory to solve the elusive structure of benzene.
One of the reasons why chemists knew that Kekulé’s model of benzene was incorrect was because the observed energy level of the molecule was much lower than the number that Kekulé would have predicted. Something else, then, was causing the energy of benzene to be lower (and thus more stable).
Pauling’s theory suggested that resonating hybrids exhibit lower energies, and ultimately he was able to use his ideas to build a structure of the molecule that fit with the energy data. Once the model was accepted, the benzene breakthrough did much to secure resonance theory as a valuable and accurate tool for understanding molecular structure.
