 Katherine Eban, “Drug Shortages: The Scary Reality of a World Without Meds,” Reader’s Digest Magazine, June 2014:
Katherine Eban, “Drug Shortages: The Scary Reality of a World Without Meds,” Reader’s Digest Magazine, June 2014:
Jennifer LaCognata, 40, a booking agent for United Airlines from Safety Harbor, Florida, first suffered an attack of night blindness in early 2011. It took months to properly diagnose her. She was vitamin A deficient, due to a shortened bowel that made her body unable to absorb fat. The good news: The problem was totally fixable with injections of a basic medicine called Aquasol A, made by Hospira, an American generic drug company.
But bad news quickly followed. LaCognata learned that manufacturing challenges had forced Hospira to stop selling the drug, effectively plunging the entire world into a shortage. Without Aquasol A, LaCognata is going blind. United Airlines placed her on unpaid medical leave because she could no longer look at a computer screen. She is forced to wear an eye patch and can see only kaleidoscopic fragments out of one eye. “My kids call me Pirate now,” she says, “instead of Mom.”
LaCognata and her husband canvassed the world for Aquasol A. They contacted the Food and Drug Administration (FDA), the State Department, and every major eye hospital in America. (They found two vials, but they were expired, so hospitals wouldn’t release them.) The couple wrote and called hospitals and organizations in Israel, China, Canada, and Brazil. LaCognata contacted charities, including the Red Cross and the Helen Keller Foundation, that serve foreign populations prone to vitamin A deficiency, without luck.
A Hospira spokesperson said that the company recognized the “critical need” for Aquasol A and had contracted with a separate manufacturing company to “accelerate the product’s return to market.” But years later, the company has yet to resume its production.
It is listed on Hospira’s website as “out of stock.”
“I can’t believe this could happen in America,” LaCognata says.
A Shocking Epidemic
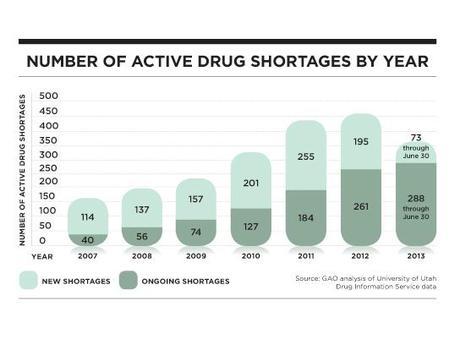
It seems unfathomable in our high-tech medical system, but in 2007, 154 drugs were in shortage, a number that almost tripled to 456 in 2012, according to the U.S. Government Accountability Office.
Threatening medical-care options and patients’ lives, drug shortages have occurred in almost every pharmaceutical category. Antibiotics, cancer drugs, anesthesia, pain control, reproductive and gynecological drugs, cardiac medicine, psychiatric drugs, and intravenous-feeding solutions have all been in varying degrees of short supply or not available at all. Recently, nitroglycerin, an emergency room staple used to treat heart attack patients, has been in such severe scarcity that its sole U.S. manufacturer has restricted hospitals to 40 percent of their usual orders. A study published this March in the Mayo Clinic Proceedings attributed more than 15 documented deaths since 2010 to either lack of treatment or the switch to an inferior drug as a result of medication shortages.
Though the FDA has scrambled to alleviate the crisis and physicians have become deft at juggling or substituting treatments, there is no comprehensive solution to this drug-supply breakdown, which has persisted for a complex array of reasons.
The first is all about money. As generic drug prices have dropped, so have manufacturers’ profits. As a result, some manufacturers have failed to invest in infrastructure and quality control. To ensure safety, the FDA has taken regulatory actions that have halted supplies, with sterile injectable drugs, such as pain meds and chemotherapy, dominating the shortage list. (These are the most complex and costly generics to make.) The business model of just-in-time manufacturing—in which companies make drugs as patients need them but do not stockpile extra—leaves no margin for unexpected events.
Noting that there are no shortages of big profit-generators, like Viagra, many practitioners and patients suspect less-than-honorable motives by drugmakers. Some experts suggest that manufacturers may have financial incentive to temporarily stop production of a drug. Medicare reform imposed certain price controls on generic drugs, but, due to a loophole, these controls are lifted if a manufacturer stops making the drug for six months.
Limited manufacturing capacity is an important factor too. Over half the drugs on the FDA shortage list had only one or two manufacturers, according to a report by the IMS Institute for Healthcare Informatics. Some blame hospital buying groups, middlemen that purchase drugs and supplies for many of the nation’s hospitals, which have awarded contracts to some manufacturers and not others, thereby suppressing competition and innovation. The result is that for any given drug, there may be only one or two generic manufacturers left to produce it, which can lead to shortages.
Predatory middlemen are making the situation even worse. A congressional investigation led by Rep. Elijah Cummings, a Baltimore Democrat, has found that shady secondary wholesalers buy up drugs in shortage and resell them, often at exorbitant prices. This explains, in part, the haphazard ebb and flow of the shortages that makes them particularly hard to handle: One day the medicine is just gone, but there’s plenty the next.
But as experts debate the cause of the shortages, there is no disagreement over their devastating impact. They’ve turned pharmacists into professional beggars and have forced doctors to change treatment protocols on the fly—in some cases, turning routine care into a roll of the dice. They’ve stopped clinical trials and have led to the suspension of the death penalty in some states that use lethal injection.
Shortages are leading hospitals and patients to get drugs from less regulated and potentially less safe sources, such as drug compounders, specialty pharmacies that mix medications for individual patients. Some compounding pharmacies, which are not subject to regulation as stringent as that for drug manufacturers, have taken advantage of this and started churning out large volumes of drugs. But this can lead to safety issues. Such dangers became vividly clear in 2012, after contaminated steroids from the New England Compounding Center led to an outbreak of meningitis that killed 64 patients. A 2013 report by the Health and Human Services inspector general found that drug shortages have led 68 percent of U.S. hospitals to turn to compounders to make versions of medicine in short supply.
Patients today have to cope not only with being sick or choosing between treatment options but also with the possibility that the drug they need may be available solely from a risky source—or not at all.
Those with life-threatening diseases have been hit hardest by shortages, in part because many of their medications have no substitute and their exacting treatment regimens cannot be delayed.
Justine Zirbes, 33, a TV producer in Minneapolis, was seven months pregnant with twins in October 2010 when she learned that her three-year-old, Axel, had leukemia. The distressing news sent her into early labor, and she was confined to bed.
As her son embarked on grueling chemotherapy for a disease that can often be cured if treated—but is almost certainly fatal if not—Zirbes learned that a national shortage of the chemotherapy drug cytarabine would affect his regimen. Doctors offered a drug called clofarabine, which was not standard treatment. Though still pregnant and on bed rest, Zirbes flatly refused. “How is this possible, in this country in 2011, that these lifesaving drugs are not widely available?” Zirbes recalls. “I was beside myself with disbelief.”
Zirbes was justified in her concern. According to a 2012 study in the New England Journal of Medicine, drug substitutions due to shortages led to higher relapse rates among children with an otherwise curable form of lymphoma.
Like Jennifer LaCognata, Zirbes embarked on a quest to find the right medicine. She contacted her senator Amy Klobuchar (D-MN), who took up the cause with proposed legislation. Zirbes produced two news segments on children affected by drug shortages. She worked every connection she had and looked as far away as Europe to find her son’s drug. Ultimately, the day before Axel was due for treatment, the hospital got enough cytarabine to treat him and another child.
Axel was reasonably lucky. Other patients, like Carey Fitzmaurice of Bethesda, Maryland, almost certainly suffered recurrence of their cancer because of drug shortages.
In 2006, Fitzmaurice, 37, was happily married with two young children and a job she loved as a policy analyst at the Environmental Protection Agency, when she learned that she had ovarian cancer and a BRCA1 genetic mutation that had likely caused it.
Over five years, she bravely underwent debilitating treatment, a recurrence of her ovarian cancer, an additional diagnosis of breast cancer, and a radical mastectomy. In the middle of 2011, things finally seemed to be breaking her way. Her breast cancer was in remission, and a chemotherapy drug called Doxil, made by a contractor for the Johnson & Johnson subsidiary Janssen, Inc., appeared to be vanquishing a recurrence of the ovarian cancer. But in August 2011, she learned there was not enough Doxil to complete her treatment.
Fitzmaurice assumed that she would be able to find some on her own. “I work for the federal government,” she says. “A lot of what I do is help people cut through red tape and find solutions. That’s how I tackled cancer to begin with: find out who the right doctor is, where to get surgery.”
But the shortage of Doxil thwarted her every effort. After four months without the medication, her ovarian cancer returned.
In a recent study from the University of Pennsylvania presented at the 2013 annual meeting of the American Society of Clinical Oncology, 83 percent of oncologists and hematologists said they’ve faced cancer drug shortages, and of those, nearly all said their patients’ treatment had been affected by drug shortages.
At the Ohio State University Comprehensive Cancer Center in Columbus, Ohio, Ryan Forrey, associate director for pharmacy and infusion services, says that in 2012, of the 60,000 doses of chemotherapy administered intravenously at his facility, almost 35 percent were affected by shortages. Treatment was interrupted or canceled, patients were switched to alternative drugs, or an alternative supply for the needed drug had to be found. His overwhelmed staff “was forced to beg, borrow, plead to get drugs for patients,” he says.
Now, whenever a patient begins chemotherapy, Forrey’s hospital sequesters the entire treatment regimen, which can be months of medication, to ensure that it is available. But Forrey is not optimistic that the drug shortages will ease.
“Every time I think it can’t get worse, it does get worse,” he says.
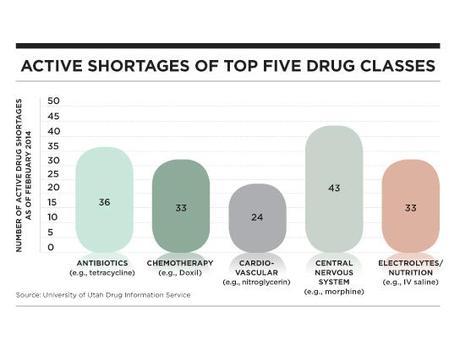
Shortages are not limited to drugs for cancer or uncommon diseases. Experts fear that scarcity of the heart attack drug nitroglycerin is endangering patients’ lives. Last spring, the most basic of antibiotics, doxycycline, used for everything from acne to Lyme disease, disappeared from pharmacies. Even intravenous saline solution, a hospital staple, has been in short supply, leading some hospitals to ration their use. In fact, almost every U.S. hospital has faced a lack of basic medicine, found an American Hospital Association survey. Many have even hired a full-time staff person specifically to navigate shortages.
Hospitals have developed complex formulas to help ration existing drug supplies—essentially, to determine which patients get medication and which don’t.
“No doctor wants to prioritize,” says Richard Schilsky, MD, chief medical officer of the American Society of Clinical Oncology. “But if you have five patients and only three vials, that’s a very real problem.”
A significant shortage creep is affecting mainstays of reproductive and sexual-health medicine, from anesthetics used in gynecologic surgery to antinausea drugs for pregnancy to antibiotics for sexually transmitted diseases, says Michele Curtis, MD, an ob-gyn in Houston. “I work in the most expensive health-care system in the world, and I am being asked to do it in third world conditions,” she says.
Thirty-seven of the 38 different components used in intravenous nutrition bags have been in fluctuating shortage since spring 2009, which is a serious threat to our country’s youngest and most fragile children. Insufficient zinc, for example, has led to raw blistering sores on the tiny hands of premature infants.
“We have been compromising what we feel is optimal care for close to three years now, and we don’t really see any end in sight,” says Jay M. Mirtallo, immediate past president of the American Society for Parenteral and Enteral Nutrition.
Rolling the Dice
Doctors, patients, and regulators have jury-rigged fixes with their own hazards, using drugs that may have worse side effects or less favorable outcomes or come from unsavory wholesalers or less regulated drugmakers.
The shortages have forced Susan Agrawal, in Chicago, to make difficult decisions as she struggles to keep her nine-year-old daughter, Karuna, alive. Karuna was born with cerebral palsy due to a ruptured umbilical cord. She has since developed an autoimmune disease that causes her organs to fail. She survives by getting her nutrients intravenously and needs bags of liquid nutrition with 14 components, which Agrawal gets from a specialized sterile infusion facility.
Agrawal has had to canvass local pharmacies and drug compounders to find the ingredients that the facility can’t get. In fact, she’s even purchased drug components from a compounding pharmacy that has had repeated run-ins with state regulators for unsanitary conditions. Then she must reconstitute the components, from powder to liquid, at her dining room table, with no guarantee of sterility, and add them to her daughter’s IV bags. “It’s like, ‘Cross your fingers,’ ” she says.
Although Karuna is very sick, the fact that her IV nutrition—which shouldn’t be this complicated—is so fraught may be compromising the quality of life she has left. Agrawal fears that given her daughter’s fragile health and immune system, the shortages “will surely cause her death.”
In a survey of 1,800 health-care practitioners, about 25 percent reported errors due to shortages. Among the 1,000 incidents: a patient who died from an infection that could have been treated had the antibiotic amikacin been available, accidental overdoses of alternative drugs, and prolonged hospitalizations due to side effects from alternative drugs.
A medicine substitution sickened Sharon Brown, who was being treated for stage 2 breast cancer at Ohio State University. She was suffering relatively few side effects from her chemotherapy drug, Taxol, but then in June 2011, her doctors were unable to procure her next dose and switched her to a similar drug, Taxotere.
Two days after her first dose, she suffered a devastating reaction, an uncommon but known side effect: She grew dizzy, broke into a cold sweat, and could not lift her arms over her head. She was so dehydrated that hospital staff could not even draw blood from her veins. For the next two weeks, she needed IV fluids every other day just to treat her dehydration. She was too sick to resume chemotherapy for another month.
Even the FDA has been boxed into a corner. After the cancer drug Doxil became unavailable in late 2011, the FDA made an emergency provision to allow an Indian generic-drug company, Sun Pharma Global, to temporarily export a similar generic drug, Lipodox, which is not approved in the United States. Although patients and doctors applauded the move, Sun Pharma has faced repeated past FDA sanctions for poor quality. (In 2009, U.S. marshals raided its U.S. manufacturing plant and shut down production.)
Patients say that drugmakers have forced them into unacceptable treatment. Sufferers of Fabry disease, a rare life-threatening enzyme disorder that dangerously slows blood supply, have only the drug Fabrazyme, made by the company Genzyme, to treat their disease. But in 2009, Genzyme was forced to shut down its manufacturing plant in Allston, Massachusetts, after a viral contamination was discovered.
As Genzyme entered into a consent decree with the FDA and struggled to open a new plant, it established a rationing program. Patients would be required to take a reduced dose instead of their whole dose. If they refused, they would be given no medicine and placed at the bottom of the waiting list. A spokesperson for Genzyme says the company imposed this condition after it consulted with a working group that included doctors and patient advocates. However, patients say that the FDA did not study the impact of the diminished dose, and—more shocking—the European Medicines Agency, Europe’s main drug regulator, found that a reduced dose accelerated disease in some patients. (Genzyme spokeswoman Lori Gorski says that during the shortage, the FDA did permit patients to take an alternative drug, Replagal, made by Shire.)
In February 2012, a Pittsburgh patent lawyer, Allen Black, PhD, who had worked as a drug developer, filed a lawsuit against the FDA on behalf of patients affected by drug shortages. Those included patients with Fabry disease as well as Jennifer LaCognata, who needs Aquasol A. The lawsuit alleged that by allowing drug companies to stop making a drug, the FDA effectively allows them to make life-or-death decisions for patients. As Black says, “There’s no appeals process. You get denied a drug? Tough.” The FDA’s response offered little comfort to patients expecting help. In a motion to dismiss the lawsuit, the FDA stated that while it “works with manufacturers to help prevent and mitigate these shortages, the FDA’s authority to address potential and actual drug shortages is limited.” Last November, the court dismissed the case.
For Real Solutions, We Need Big Reform
In July 2012, Congress passed the Food and Drug Administration Safety and Innovation Act, which included provisions originally spearheaded by Justine Zirbes’s senator Amy Klobuchar. The law requires that drugmakers give the FDA early notification of any manufacturing issues or business decisions that may lead to shortages. It requires the FDA to expedite inspections and reviews of manufacturing sites that could help resolve shortages.
There is some evidence that the FDA has been successful in holding back the tide. In 2013, the number of new drugs added to the shortage list was far less than the number added in 2012. But in the first quarter of 2014, active shortages remained close to the highest level ever. The situation, which was the subject of a congressional hearing in February, remains “very critical,” says Erin Fox, director of the University of Utah’s drug information service.
Critics say the FDA’s response is mere window dressing that has done little to change underlying problems. “It’s fine to say that the FDA should have six months’ advance notice,” says Dr. Schilsky. But he views the new legislation as “doing nothing to address the root causes” of the problem.
Meanwhile, doctors and patients have little information about when they can expect shortages to be resolved. Despite planning and promises from drugmakers, “many resolution dates are unknown or unmet,” says Ryan Roux, chief pharmacy officer at the Harris County Hospital District in Texas.
Companies aren’t penalized for drug shortages or incentivized to avoid them, experts say. The only real solution, say a number of health-care professionals, is to require drug manufacturers to stockpile medicine and to ensure that more than one drugmaker produces it. “There needs to be a way to obligate multiple manufacturers to make these lifesaving medications,” says Ohio State’s Forrey.
The FDA would have to require that manufacturers change their just-in-time manufacturing model and set aside reserves of lifesaving drugs, which the FDA has claimed is impossible. But one executive at a pharmacy benefit-management company, who asked to remain anonymous, says the FDA could easily mandate this: “Don’t give me this stuff: ‘We can’t tell the drug companies what to do,’” he says. “Yes, you can; you do it all the time.” The FDA, with all its leverage, “could probably solve 90 percent of the problem.”
Some vital industries, such as technology and defense, encourage what is called second sourcing, in which manufacturers may sublease at least one fifth of production to backup companies to avoid supply-chain disruptions in the event of natural or other disasters. Justine Zirbes, who faced the prospect that her son Axel could have died from leukemia without cytarabine, says that manufacturers who “stop making a lifesaving drug” should be subject to criminal prosecution.
In a separate proceeding, Jennifer LaCognata sued Hospira on the grounds that the company failed to plan for contingencies in its shortage of Aquasol A. Last June, Florida’s 11th Circuit Court of Appeals dismissed the suit. Her lawyer, Allen Black, then petitioned the U.S. Supreme Court, which declined to take the case. LaCognata, whose vision continues to deteriorate and who lost her house in a foreclosure proceeding, says she has to remain strong for her kids. “I have to have a very upbeat attitude,” she says, “or it would just consume [me].”
Just recently, her lawyer was staggered to learn from a friend who works for Doctors Without Borders that a version of the vitamin A drug that LaCognata needs is being manufactured in France, sold under the name Nepalm Vitamin A. But with her financial resources depleted, her doctor reluctant to prescribe it, and her need to get a compassionate-use exemption from the FDA (so the drug can get through customs), LaCognata just has to figure out how to obtain it.

