Reminder: Pharmboy is available to chat with Members, comments are found below each post.
Over a year ago, I noted that the winds of change were coming for the pharmaceutical industry. This week, a very good article came out in the Guardian that stated two of the largest firms in the business, GSK and AZN are getting out of the neuroscience business. This is a huge blow to those that suffer from mental disorders such as bipolar, depression, anxiety, schizophrenia, OCD and the likes. The reason for this, according to David Nutt, a professor of Neuropsychopharmacology at Imperial College in London, is:
Despite the public health imperative, not only has EU research funding remained very low, but – even worse – big pharma is increasingly coming to see research into better neuropsychiatric drug targets as economically non-viable.
There are a number of reasons why companies are leaving the field, according to the report. Medicines for brain disorders take longer to develop than for other conditions – on average, 13 years – and there is a high failure rate.
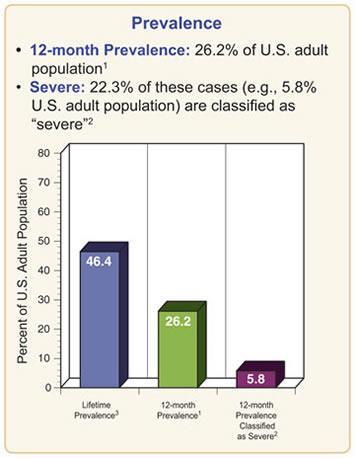
Figure 1. Prevalence of Mental Disorders in the US from NIMH.
The brain is one of the last unknowns of medicine. It is a complex network of cells that interact to enable humans to hear, see, talk, feel, remember, experience emotion, and it controls our ‘biological clocks.’ I believe that the brain will be the ultimate frontier for personalized medicine. Mental disorders need to be treated on an individual basis. There is nothing worse than having a loved one or someone very close to you who suffers from these debilitating disorders. The patients require time with a psychiatrist/psychologist to be properly diagnosed. The varying treatment regimens take time, and in many cases patients will have to go on and off multiple medications until the right combination and dosages are attained. The drug combinations can take years to complete and the patient must want to get better and those around must provide support and compassion that the patient requires for successful treatment. Further, those close to the patient must try to understand that it is very hard for the patients to describe, with words, what they are experiencing.
The most important things are the hardest things to say. They are the things you get ashamed of, because words diminish them – words shrink things that seemed limitless when they were in your head to no more than living size when they’re brought out. But it’s more than that, isn’t it? The most important things lie too close to wherever your secret heart is buried, like landmarks to a treasure your enemies would love to steal away. And you may make revelations that cost you dearly only to have people look at you in a funny way, not understanding what you’ve said at all, or why you thought it was so important that you almost cried while you were saying it. That’s the worst, I think. When the secret stays locked within not for want of a teller, but for want of an understanding ear.
– Different Seasons, Stephen King
It is my hope that we will not have the ‘dead’ space in the neuroscience area for 20-30 years! Mental health is an under-recognized, under treated area of medicine with a very large potential market. Remember this: when funding is cut from NIH, it hurts those in the sciences who are looking for the answers!
There are many good medications out there, many are generic including Lamictal, SSRI’s (Zoloft), tricyclic antidepressants, benzodiazepines (Ativan), mood stablizers (Tegretol) and many, many more. Because of the lag time for many of these agents to work, more research is needed to help understand the complex interactions of the neuronal network. There are a few companies focused on receptors in the brain for the treatment of mental disorders, and one company who is working in the mental disorders area is highlighted below.
Brought up in chat by a member, Targacept (TRGT) and AstraZeneca are co-developing TC-5214, the S-(+) stereoisomer of the nicotinic acetylcholine receptor (nAChR)-antagonist, mecamylamine, for the potential oral treatment of major depressive disorder (MDD). The company is also investigating the R-(-) stereoisomer of mecamylamine as TC-5213. From clinicaltrials.gov, TRGT has several Phase 3 trials ongoing:
- In September 2010, a multicenter, randomized, double blind, placebo controlled, parallel group, phase III trial began in Europe to assess the safety and efficacy of three doses of TC-5214 as an adjunctive therapy in patients with MDD (expected number of patients is 2200+). The primary endpoint was change in the Montgomery-Asberg Depression Rating Scale (MADRS) total score at week 16. The study is due to complete in January ’12.
- In August 2010, a multicenter, randomized, double blind, placebo controlled, parallel group, phase III study began in Europe to assess the safety and efficacy of flexible doses of TC-5214 as an adjunctive therapy in patients with MDD (expected number of patients is 900+). The primary endpoint was change in MADRS total score at weeks 8, 9, 10, 12, 14 and 16. The study is due to complete in August ’11.
- In June 2010, a multicenter, randomized, double blind, placebo controlled, parallel group, phase III study began in the US and Puerto Rico to assess the safety of TC-5214 as an adjunctive therapy in patients with MDD (expected number of patients is 2000). The primary endpoints are safety and tolerability.
- In June 2010, a multicenter, randomized, double blind, placebo controlled, parallel group, phase III study began in the US to assess the safety and efficacy of three doses of TC-5214 as an adjunctive therapy in patients with MDD (expected number of patients is 2200+). The primary endpoint was change in MADRS total score at week 16. The study is expected to complete in November ’11.
I will consider buying some long dated options in TRGT, so stay tuned in chat for this.
++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++++
I feel very strongly about NIH funding and the roles that it has played in our quest for understanding medicine. If this country is to continue in its ways and remain on the leading edge of research, teaching and funding in the sciences is necessary. My reasons for bringing this up is goes to my last article here on personalized medicine, and this topic will start being a focus for us in the years to come. DNDN, ONTY, NWBO, IMUC, and even FCSC that are all using cells from self, altering them and putting them back into the person for different diseases/modalities. For now, though, I remain focused in the cancer arena, were the biggest bang for buck continues to develop.
Several PSW articles have focused on cancer, one of the largest engine drivers of the near future.
We have focused on big companies (NVS, SNY, BMY), mid-tiered companies (BIIB, ONXX, Roche) and those on the leading edge (IMGN, SGEN, CRIS, ONTY). I have a few that I would like to add to our list of possible stocks in the coming months. A few were noted here and I reiterate IMGN, ARIA, SGEN, CRIS, NSPH and MITI as positions I like for a longer term hold.
Other potential companies I am considering and are now on my watch list are:
Incyte (INCY) & YM Biosciences (YMI) – both companies are competing in the space of JAK inhibitors for the treatment of myleofibrosis. Both compounds are similar in nature, but in early trials, YMI’s compound CYT387, aids in the treatment of anemia, a common occurance in myleofibrosis. YMI’s data is very early in clinical trials, so it remains unclear if this is a true differentiator in the treatment of this cancer, but what if? Currently, INCY is teamed up with NVS, and the company has asked for a priority review for their drug ruxolitinib. YMI has more data due out at years end, so I like buying the stock in here and selling a few puts and calls into the year end run up. INCY will be put on a watch list, as I am concerned about its high price for one disease treatment (market cap of >$2B).
Ziopharm Oncology (ZIOP) – is an oncology company with several drugs at various stages of clinical trials.
- Darinaparsin (from MDA Cancer Institute): in both IV and oral formulations, darinaparsin (Zinapar) is one of a series of small-molecule organic arsenic-derived apoptosis stimulators, for the potential treatment of cancer, including solid tumors and myeloma. They have Orphan Status for peripheral T-cell lymphoma (PTCL) and data are expected in late 2011 for this Phase 2 trial.
- Palifosfamide (Zymafos) is the lead bifunctional DNA alkylator and cross-linker in a series of pre-activated forms of ifosfamide, for the potential iv treatment of ifosfamide-responsive tumor types, such as soft tissue sarcoma (STS). The compound is in Phase 3.
- In collaboration with Intrexon, ZIOP is developing a combination regimen using intratumorally injected autologous dendritic cells adenovirally transduced to inducibly express human IL-12 (hIL-12) when activated by an oral small-molecule agent. Basically, the cells are from self, infected with a virus, and the virus is turned on using a drug specifically made for the virus. IL-12 is a very potent anti-angiogenic (think Celgene). This is a very neat trick, and could be used for melanoma and solid tumors. Time will tell for this technology.
Stay tuned in chat for possible entry positions in these stocks and the others noted above.

