A few months have passed since my last post, as there has been things happening in the biotech world, but nothing 'new' enough to jump on board and get behind. Drug development is a long, arduous process, and clinical trials are the worst. The smaller the diseases are in a population, the longer it takes to enroll, test and then post results.
At PSW, we have been patient investors in MITI (bought by Amgen), CRIS, EXEL, SGEN and many others, and waiting in the wings are CLDX, YMI, and now PGNX. I noted several years ago, that cancer and neuroscience are the final frontiers to conquer, and they remain today where they were then…so time does not change things that fast! Diabetes and cardiovascular diseases are pretty well served with existing therapies, and yes, there will be a few that creep into the space, but overall these diseases needs are met. The hottest areas in pharma today remain small, elusive, and lucrative diseases that demand a premium – cancer and fibrosis.
Fibrosis is another topic, and in the next few months, an article or two will go over some new therapies for fibrosis, which can manifest itself in several different disease types (IPF, kidney, scleroderma, etc). For now, this article will focus on cancer treatments, and more specifically, monoclonal antibodies (mAbs) that have attached to them a chemotherapeutic.
First, a short summary on mAbs and how they work.
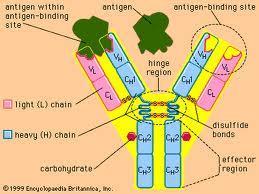
Antibodies are produced by B-cells that is used by the immune system to identify and neutralize foreign objects such as bacteria and viruses. There are several types of antibodies made for the defense of the body, and they can be read about here.
Scientists figured out how to manufacture antibodies to a specific antigen in the late 1970s, but it was not until the '80s that the use became more feasible. Mouse hybrids were the first antibodies used (called chimeric suffix -ximab), and now there are humanized (suffix -zumab) and fully human mAbs (suffix -umab). Below is a partial list of approved mAb therapies.
(Targeted disease)
Abciximab ReoPro Eli Lilly 1994 chimeric inhibition of glycoprotein IIb/IIIa Cardiovascular disease
Adalimumab Humira Abbot 2002 human inhibition of TNF-α signaling Several auto-immune disorders
Alemtuzumab Campath Genzyme 2001 humanized CD52 Chronic lymphocytic leukemia
Basiliximab Simulect Novartis 1998 chimeric IL-2Rα receptor (CD25) Transplant rejection
Belimumab Benlysta GlaxoSmithKline 2011 human inihibition of B- cell activating factor Systemic lupus erythematosus[disambiguation needed]
Bevacizumab Avastin Genentech/Roche 2004 humanized Vascular endothelial growth factor (VEGF) Colorectal cancer, Age related macular degeneration (off-label)
Brentuximab vedotin Adcetris 2011 Chimeric CD30 Anaplastic large cell lymphoma (ALCL) and Hodgkin lymphoma
Canakinumab Ilaris Novartis 2009 Human IL-1β Cryopyrin-associated periodic syndrome (CAPS)
Cetuximab Erbitux Bristol-Myers Squibb/Eli Lilly/Merck KGaA 2004 chimeric epidermal growth factor receptor Colorectal cancer, Head and neck cancer
Certolizumab pegol[19] Cimzia UCB (company) 2008 humanized inhibition of TNF-α signaling Crohn's disease
Daclizumab Zenapax Genentech/Roche 1997 humanized IL-2Rα receptor (CD25) Transplant rejection
Denosumab Prolia , Xgeva Amgen 2010 Human RANK Ligand inhibitor Postmenopausal osteoporosis , Solid tumor`s bony metasteses
Eculizumab Soliris Alexion Pharmaceuticals 2007 humanized Complement system protein C5 Paroxysmal nocturnal hemoglobinuria
Efalizumab Raptiva Genentech/Merck Serono 2002 humanized CD11a Psoriasis
Gemtuzumab Mylotarg Wyeth 2000 humanized CD33 Acute myelogenous leukemia (with calicheamicin)
Golimumab Simponi Johnson & Johnson/Merck & Co, Inc. 2009 Human TNF-alpha inihibitor Rheumatoid arthritis, Psoriatic arthritis, and Ankylosing spondylitis
Ibritumomab tiuxetan Zevalin Spectrum Pharmaceuticals, Inc. 2002 murine CD20 Non-Hodgkin lymphoma (with yttrium-90 or indium-111)
Infliximab Remicade Janssen Biotech, Inc./Merck & Co 1998 chimeric inhibition of TNF-α signaling Several autoimmune disorders
Ipilimumab ( MDX-101 ) Yervoy 2011 Human blocks CTLA-4 Melanoma
Muromonab-CD3 Orthoclone OKT3 Janssen-Cilag 1986 murine T cell CD3 Receptor Transplant rejection
Natalizumab Tysabri Biogen Idec/Élan 2006 humanized alpha-4 (α4) integrin, Multiple sclerosis and Crohn's disease
Ofatumumab Arzerra 2009 Human CD20 Chronic lymphocytic leukemia
Omalizumab Xolair Genentech/Novartis 2004 humanized immunoglobulin E (IgE) mainly allergy-related asthma
Palivizumab Synagis MedImmune 1998 humanized an epitope of the RSV F protein Respiratory Syncytial Virus
Panitumumab Vectibix Amgen 2006 human epidermal growth factor receptor Colorectal cancer
Ranibizumab Lucentis Genentech/Novartis 2006 humanized Vascular endothelial growth factor A (VEGF-A) Macular degeneration
Rituximab Rituxan, Mabthera Biogen Idec/Genentech 1997 chimeric CD20 Non-Hodgkin lymphoma
Tocilizumab ( or Atlizumab ) Actemra and RoActemra 2010 Humanised Anti- IL-6R Rheumatoid arthritis
Tositumomab Bexxar GlaxoSmithKline 2003 murine CD20 Non-Hodgkin lymphoma
Trastuzumab Herceptin Genentech 1998 humanized ErbB2 Breast cancer
1 Waldmann, Thomas A. (2003). "Immunotherapy: past, present and future". Nature Medicine 9 (3): 269–277. doi:10.1038/nm0303-269
With another twist on mAb treatments, companies figured out how to target cancer cells, and then deliver a 'payload' to the cell of chemotherapeutics. Immunogen (IMGN) and Seattle Genetics (SGEN) are two companies who developed the TAP ( tumor-activated prodrug) and ADC (antibody drug conjugate) technologies, respectively. SGEN has been very active in this area, and has licensed their technology out to several other companies including Celldex (CLDX) and Progenics (PGNX).
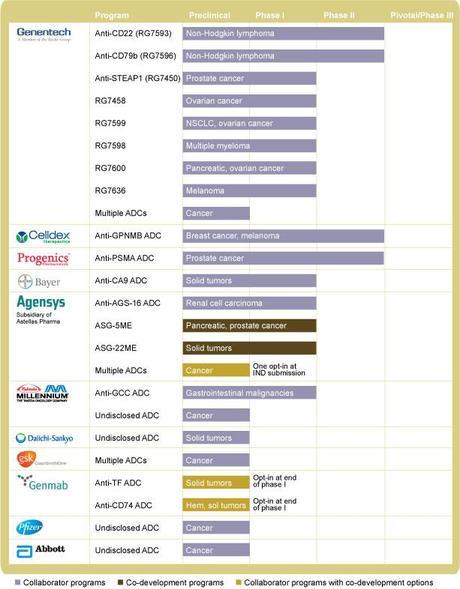
Celldex (CLDX) – the company has two late stage candidates for the treatment of breast cancer (CDX-011) and glioblastoma (Rindopepimut). Rindopepimut is Celldex's most advanced candidate and being investigated for glioblastoma. The drug is an intradermal injectable peptide vaccine targeted against epidermal growth factor receptor (EGFR) vIII developed by John's Hopkins. In its previous trial, it showed significant progress in overall survival and progression-free survival. However, with the company enrolling patients, there is not a great deal of news surrounding this candidate. CDX-011 (glembatumumab vedotin), in its Phase 2b EMERGE study in patients with glycoprotein NMB (GPNMB) expressing, advanced, heavily pretreated breast cancer, impressive response rates compared to current, available therapies in patients with advanced, refractory breast cancers with high GPNMB expression (expression in e25% of tumor cells). Final data are to be released any time, and taking some profits off the table now. Selling some puts if they pull back is a very wise way to play for a good longer term hold. For a risk on trade, selling the Dec $5 puts and buying the $7/8 BCS for a net 10 c on the $1 spread offers a bit of a risk reward profile that warrants some consideration.
Progenics (PGNX) – the company's main drug (Relistor) is used for relieving the constipation side effects of patients who are taking opioids (e.g., morphine) for pain. While this is a large market, the drug is given by subcutaneous injection, and it was delayed by the FDA (cosponsored by Salix). This delay, while a set back, should only be about 18 months or so. The more compelling case in PGNX's pipeline is their ADC mAb. PSMA ADC, an antibody-drug conjugate ("ADC") therapy for prostate cancer that binds to PSMA, a protein that is abundantly expressed on the surface of prostate cancer cells, as well as cells in the newly formed blood vessels of major solid tumors. The drug that is conjugetd to the mAb is monomethyl auristatin E, the same drug that is used in SGEN's Adcetris (brentuximab vedotin).
In the initial phases of the Phase 1 trail, four of nine patients treated with PSMA-ADC at 1.8 mg/kg showed reduced prostate specific antigen, circulating tumor cells and/or bone pain. Subsequent patients have shown that PSA reductions of 50% or more, and CTC (circulating tumor cells) reductions of less than 5 cells/7.5 ml blood were seen in around 50% of patients at doses of greater that 1.8 mg/kg. While it is early on, the mAb drug complex is compelling enough for a small allocation.
- Pharm

