A difficult concept for my students is the determination of polarity based on the difference of electronegativity of individual bonds in a molecule. Out of the four textbooks I've used for this topic in class, only one of them really includes illustrations, diagrams and tables that are effective in conveying this topic with any kind of logic.
Because students have trouble building the models of the molecules, naturally it is hard for them to imagine the dipole moments created by individual bonds within the molecule. These dipole moments are directional and have magnitude. Therefore, in the case of a symmetrical molecule, these directional dipole moments cancel each other out and you actually get a nonpolar molecule.
Examples listed below:
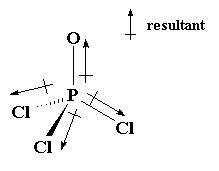
(PCl3O)This is a picture of an electron geometry of tetrahedral (four groups surrounding central atom) that also has a molecular shape of a tetrahedral. In many cases this shape is perfectly symmetrical and the individual dipole moments cancel each other out. In this case, the molecule is not symmetrical because the atoms surrounding the phosphorus have different electronegativity values. As noted, the net dipole moment is pointing toward the oxygen atom and the molecule is considered polar.
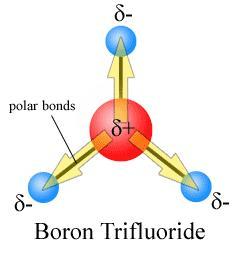
(BF3) Boron trifluoride is an example of a trigonal planar electronic geometry that also has trigonal planar molecular shape (3 groups surrounding central atom with 0 lone pairs). In this case, all atoms surrounding the central atom have the same electronegativity values (same atom) and therefore the individual dipole moments of each bond are the same. If we added the sum of the vectors the sum would be zero. Therefore, the molecule has no overall dipole moment and is considered a nonpolar molecule.
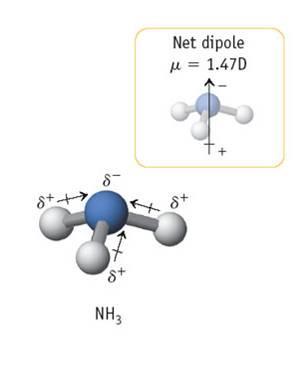
(NH3) This ammonia molecule is a perfect example of the tetrahedral electron geometry with the trigonal pyramid molecular shape. Because of the lone pair of electrons on the nitrogen, the vectors pointing from the hydrogen to the nitrogen sum to be a net dipole moment pointing straight up through the molecule. As noted, the net dipole is 1.47 D with the magnitude shown. This is not a symmetrical molecule and in most cases exhibits an overall dipole moment.
**An exception to the rule that a tetrahedral/pyramid is polar would be PH3. While it has the same shape as NH3 it is a nonpolar molecule. The reason is that there is no difference in electronegativity between phosphorus and hydrogen (calculated difference). Therefore, the asymmetry of the molecule does not matter- there is no dipole.
 The water (H2O) molecule to the left is a tetrahedral electron geometry (4 group surrounding central atom) with a bent shape. The two lone pairs are not shown in this drawing. The four total groups (2 bonding and 2 lone pairs) assume the usual tetrahedral structure, however visually only two atoms jut out from the central atom. This creates the bent shape. The dipole moment of this molecule is similar to that of NH3 in that it points straight up through the molecule. If you add the vectors of a dipole pointing toward the oxygen from each hydrogen, you get a straight line through the center.
The water (H2O) molecule to the left is a tetrahedral electron geometry (4 group surrounding central atom) with a bent shape. The two lone pairs are not shown in this drawing. The four total groups (2 bonding and 2 lone pairs) assume the usual tetrahedral structure, however visually only two atoms jut out from the central atom. This creates the bent shape. The dipole moment of this molecule is similar to that of NH3 in that it points straight up through the molecule. If you add the vectors of a dipole pointing toward the oxygen from each hydrogen, you get a straight line through the center. 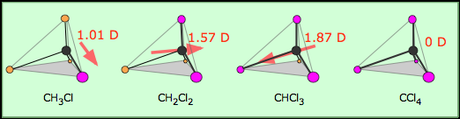 The four tetrahedral molecules to the left (variations of methyl/chlorine molecules) exhibit the change of a dipole depending on how many electronegative substituents are attached to the central atom. With one chlorine (far left) the dipole moment points in the direction of the one polar bond. For two chlorines, the overall dipole points halfway in between the vectors. On the far right, notice that the dipole is 0D. This means that when carbon has four chlorines surrounding it in a tetrahedral arrangement, the individual dipole moments cancel out and the molecule has no polarity. Carbon tetrachloride is an example of a tetrahedral molecule that has polar bonds but no overall dipole moment or polarity.
The four tetrahedral molecules to the left (variations of methyl/chlorine molecules) exhibit the change of a dipole depending on how many electronegative substituents are attached to the central atom. With one chlorine (far left) the dipole moment points in the direction of the one polar bond. For two chlorines, the overall dipole points halfway in between the vectors. On the far right, notice that the dipole is 0D. This means that when carbon has four chlorines surrounding it in a tetrahedral arrangement, the individual dipole moments cancel out and the molecule has no polarity. Carbon tetrachloride is an example of a tetrahedral molecule that has polar bonds but no overall dipole moment or polarity.
