The aging of our bodies is by definition cellular aging, and it is hard to keep track with all the theories on why cells age. One of the most venerable models is that increasing damage to energy producing mitochondria in cells is a fundamental cause of cell decay and death. Mitochondrial energy production is accompanied by a low level of the aberrant production of reactive oxygen species (ROS) that damage mitochondria DNA and proteins. Another model suggested by Sinclair and colleagues is that alterations in nuclear gene expression due to reduced activity of the deacetylase SIRT1 may be the culprit. (SIRT1 is the enzyme linked to the anti-aging activity of resveratrol). Increasing this activity by increasing NAD+ (the energy coenzyme Nicotine Adenine Dinucleotide) levels can reverse age-dependent mitochondrial dysfunction. Here are highlights and the summary of their article.
Highlights
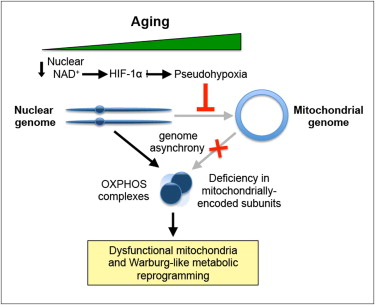
-A specific decline in mitochondrially encoded genes occurs during aging in muscle
-Nuclear NAD+ levels regulate mitochondrial homeostasis independently of PGC-1α/β
-Declining NAD+ during aging causes pseudohypoxia, which disrupts OXPHOS function
-Raising nuclear NAD+ in old mice reverses pseudohypoxia and metabolic dysfunction
Summary
Ever since eukaryotes subsumed the bacterial ancestor of mitochondria, the nuclear and mitochondrial genomes have had to closely coordinate their activities, as each encode different subunits of the oxidative phosphorylation (OXPHOS) system. Mitochondrial dysfunction is a hallmark of aging, but its causes are debated. We show that, during aging, there is a specific loss of mitochondrial, but not nuclear, encoded OXPHOS subunits. We trace the cause to an alternate PGC-1α/β-independent pathway of nuclear-mitochondrial communication that is induced by a decline in nuclear NAD+ and the accumulation of HIF-1α under normoxic conditions, with parallels to Warburg reprogramming. Deleting SIRT1 accelerates this process, whereas raising NAD+ levels in old mice restores mitochondrial function to that of a young mouse in a SIRT1-dependent manner. Thus, a pseudohypoxic state that disrupts PGC-1α/β-independent nuclear-mitochondrial communication contributes to the decline in mitochondrial function with age, a process that is apparently reversible.

