
The author in his laboratory at the Linus Pauling Institute of Science and Medicine. Originally published in Science Digest, June 1986.
[Guest post written by John Leavitt, Ph.D., Nerac, Inc., Tolland, CT.]
In 1987, my colleagues at the Pauling Institute in Palo Alto, colleagues at Stanford and I published a paper that clearly demonstrated that expression of a charge-altered mutant human beta-actin (glycine to aspartic acid substitution at amino acid 245; G245D) caused non-tumorigenic, immortalized human fibroblasts to form aggressive tumors in nude mice (Leavitt et al, 1987a). When these tumor-derived cells were examined, we discovered that they exhibited further elevated expression of the mutant beta-actin and these tumor-derived cells formed tumors even more rapidly – observations that were consistent with the role of this mutation in the tumorigenic phenotype. Furthermore, over-expression of mutant beta-actin was associated with down-regulation of three abundant tropomyosin isoforms in a well-documented transformation-sensitive manner (Leavitt et al, 1986; Leavitt et al, 1987a and Ng et al, 1988). These final papers were the culmination of research conducted at the Linus Pauling Institute of Science and Medicine (LPISM) from December 1981 to March 1988.
Normally when a scientific observation is never repeated it is usually not worth remembering. In this case, twenty-six years after our 1987 publication, a study was published by Schoenenberger et al. at the Biozentrum in Basel, Switzerland, that reproduced our findings in a different cell system, a rat fibroblast model (provided to them by LPISM in 1986). Furthermore, these investigators extended our findings by characterizing new aspects of abnormal behavior of the mutant beta-actin and cells that express this aberrant protein, which help to explain its potential role in cancer such as enhancement of tumor cell motility and invasiveness.
In addition to enhancement of tumor growth and alteration of cell shape, the Swiss investigators presented the following findings to clarify and support the oncogenicity of this mutation:
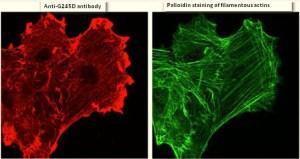
- The mutant actin stimulated formation of ruffles at the cell periphery as shown by staining of cells with an antibody that bound specifically to the mutant epitope of the mutant beta-actin (left image below)
- The mutant actin concentrated primarily in these ruffles (palloidin staining reveals the location of filamentous actin in stress fibers; right image below)
- The expression of mutant actin inhibited the tropomysin binding to filamentous actin and tropomysin did not accumulate in the ruffles
- Mutant actin colocalized with Rac1 (a GTPase mediator of membrane ruffling) and beta1-integrin (adhesion protein) in the ruffles
Back-tracking several years, the discovery of this actin mutation was made in a mutagenized cell line isolated by Takeo Kakunaga at the National Cancer Institute (NCI) in 1978. During the month that his paper was published, I walked over to NCI from my lab across the street at the Bureau of Biologics (FDA) to have a chat with Takeo about using his in vitro transformed Syrian Hamster cells as a model system to identify changes in protein expression that correlated with neoplastic transformation. After describing what I wanted to do, he seemed agreeable but then casually mentioned that he had succeed in transforming human fibroblasts into tumor forming cells. I nearly fell off my chair because human cells had never been transformed in vitro before, a major problem for cancer researchers at that time.
I blurted out that we should do the work that I had proposed in his human cell model system, comparing protein expression by the transformed neoplastic cells with their normal precursor cells. My hypothesis was that this comparison would allow identification of proteins that were turned on or turned off in expression by comparing protein profiles of the most abundant 1,000 proteins expressed in these cells and resolved by high-resolution 2-D gel separation (protein profiling). My plan was to look for charge-altering mutations in proteins that might govern neoplastic transformation and tumorigenesis. A fall-back goal was to define the pattern of qualitative and quantitative changes in protein synthesis to try and get a handle on the mysterious mechanism of human cancer development. A summary of the global changes in gene expression of neoplastic human fibroblasts was published from LPISM in 1982 (Leavitt et al, 1982).
Within two weeks, in May of 1978, I was metabolically labeling the total cellular proteins (with the amino acid S-35 methionine) of the normal fibroblasts and three strains of cell lines derived from the normal culture which were immortalized, only one of which formed subcutaneous tumors in nude mice. After four hours of labeling, I prepared extracts of S-35 methionine labeled proteins from each of the four cultures and loaded 25-microliter aliquots of each sample onto the top of clear noodle-like isoelectric focusing gels (7-inch long urea-polyacrylamide gels with the thickness of thin spaghetti) which separated the complex mixture of total cellular proteins by their net charge (isoelectric point). These gels were subjected to isoelectric electrofocusing of the proteins overnight. The next morning I harvested the spaghetti-like gels, and incubated them in a detergent that would bind to the proteins to help separate them by their molecular weights in a second dimension. So, these proteins were first denatured and separated by their net charge and then, in a second dimension, separated by their size on a thin rectangular slab gel.
After about five hours of separation in the second dimension, I was soon to learn that I had separated more than 1,000 denatured protein subunits (polypeptides) by their differing charges and molecular weights. The final step before autoradiography, which revealed the full protein profile, was to fix and stain the gels to get a glimpse of the resolution of these peptide patterns. The staining of these rectangular gels revealed only the most abundant architectural cellular proteins, the largest number of which were cytoplasmic beta- and gamma-actin, at a ratio of about 2:1 in abundance, respectively. The figure below shows what quickly appeared as the gels were de-stained. In the one tumorigenic cell line, instead of seeing a 2:1 ratio of beta- to gamma-actin, a new abundant protein at about one unit charge more negatively charged (more acidic), and half of the normal beta-actin was lost. The pixilation of these three radioactive “spots” immediately suggested to me that one of the two functional genes (alleles) encoding beta-actin had mutated, possibly due to the replacement of a neutral amino acid with a negatively charged amino acid. This prediction was no mystery to me as I had demonstrated this type of electrophoretic shift in another protein a year earlier at Johns Hopkins.
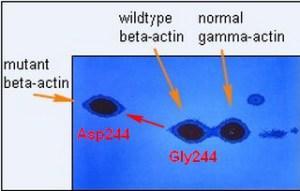
A number of experiments were done to build the case for the beta-actin mutation, and then I wrote a letter to Klaus Weber at the Max-Planck Institute in Goettingen, Germany, asking for his help in sequencing these actins. His lab was the only one in the world sequencing actins, e.g. the four muscle forms of actins. It only took Klaus two weeks to respond affirmatively, an indication to me that he was eager. I provided him with the actin proteins from this cell line and it took a postdoctoral fellow, Joel Vandekerhkove, and Klaus a little over a year to determine the complete amino acid sequences of the mutant beta-actin and both the wildtype beta- and gamma-actins, to define the mutation that had occurred. We published the result shown above in the top journal Cell in December 1980. Four years later, my colleagues at Stanford and I published my cloning of the mutant and wildtype human beta-actin gene, and the experiments that formally proved the mutation at the level of the gene (Leavitt et al, 1984). Three years after that, we published the experiments that demonstrated the tumorigenic effect of this mutation in immortalized human fibroblasts.
The dramatic nature of this discovery was never fully appreciated, perhaps, because no other actin mutations had been reported and it took Scheonenberger, et al. twenty-six years to complete the work published in September 2013. In another recent related development, Lohr et al. reported reoccurring beta-actin mutations in a panel of tumor cell samples from patients with diffuse large B-cell lymphoma.
One interesting piece of information that came out of our initial sequencing of these actins was the degree of evolutionary conservation of human beta- and gamma-actin. These two actins differ by only four amino acids at the N-terminus, whereas the four muscle-specific human isoforms are more divergent. Comparing the sequence of actin cloned from Saccharomyces cerevisiae (yeast) with these human sequences (sequences stored at the National Center for Biotechnology Information; NCBI) reveals that yeast and human cytoplasmic actins are 92% identical in their sequences (differing by only 31 amino acids out of 375) and most of these amino acid exchanges are conservative replacements both structurally and thermodynamically. This makes these actins the most highly conserved proteins (on a par with histones H3 and H4) among the 20,000 or so known human protein sequences. This fact presents an argument for the fundamental importance of non-muscle cytoplasmic actins in eukaryotic life. It turns out that among actin sequences of all species, no replacement of the Glycine 245 has ever been documented as a result of species divergence or mammalian isoform divergence.
When we introduced the mutant beta-actin gene into a non-tumorigenic immortalized fibroblast strain by gene transfer (Leavitt et al, 1987a), we isolated a transfected clone in which the ratio of exogenous mutant beta-actin to wildtype beta- + gamma-actin was 0.88 – a 76% higher level of expression than the mutant actin in the original mutated cell line in which the mutation arose (0.5 ratio). When we isolated and cultured the cells from a tumor formed by this cell line, the ratio of exogenous mutant beta-actin to wildtype beta- + gamma-actin had increased to 1.95, indicating that about 64% of the total cytoplasmic actin was the mutated beta-actin. Whereas the initial transfectant cell line produced visible tumors at about six weeks, the tumor-derived transfectant cells expressing 64% mutant actin formed visible tumors at about 1.5 weeks. Thus, expression of this mutation was not inhibitory to cell growth.
The other surprising finding was that cell lines expressing the transfected mutant actin gene did not have higher levels of cytoplasmic actins in them because the two endogenous wildtype beta- and gamma-actin genes were coordinately down-regulated (auto-regulated) so that the relative rates of total actin synthesis remained around 30% compared to S-35 methionine incorporation into 600 surrounding non-actin polypeptides in the protein profile (Leavitt et al, 1987b). This auto-regulation phenomenon was reproduced by Minamide et al. (1997) ten years later.
Cytoskeletal rearrangement of actin microfilaments, as well as changes in composition of tropomyosin isoforms and other actin-binding proteins, have long been associated with neoplastic transformation. However, before our study, causal mutations in a cytoplasmic actin had apparently not been considered. It is perhaps consistent then that Ning et al. (2014) have recently described genetically inherited polymorphisms in the actin-bundling protein, plastin (also discovered and cloned at LPISM), that significantly affect the time of tumor recurrence in colorectal cancer after resection and chemotherapy.
During my tenure at the Pauling Institute, I felt that Dr. Pauling understood and appreciated this work and its relevance to the fundamental nature of cancer development. Progress can be slow, but ultimately true understanding of cancer will emerge from this type of research…and I predict that cytoplasmic actins and actin-binding proteins that regulate actin organization and function in the cytoskeleton will be understood to play a central role in the manifestation of the tumorigenic phenotype.
