[Part 4 of 7]
“The Nature of the Chemical Bond. IV. The Energy of Single Bonds and the Relative Electronegativity of Atoms.” Journal of the American Chemical Society, September 1932.
The first three papers published by Linus Pauling in his nature of the chemical bond series were all novel, and the first paper in particular made a significant impact. But it is the fourth paper that has proven to be perhaps the most influential of all. In it, Pauling introduced his idea of the electronegativity scale, a cohesive and logical tool that proved to be of major import to the discipline.
The concept of electronegativity can be understood in terms of the likelihood that an atom will attract a pair of valence (or bonding) electrons. The more electronegative an atom is, the more likely that it will attract electrons. The most electronegative element is fluorine and the least electronegative element is francium.
Pauling was able to develop a scale for electronegativity using insights into valence bond energies. The ideas that he had put forth in his three preceding papers did not always firmly commit to either Molecular Orbital theory or Valence Bond theory, and this vacillation led to significant flaws in paper number two. Beginning with the fourth paper however, Pauling chose to base his work on the tenants of Valence Bond theory.
The electronegativity scale that Pauling developed was also quite intuitive in that it could not be calculated directly, but instead had to be inferred using the atomic and molecular properties of a given element. And even though its creation relied upon a series of assumptions and simplifications, the tool was nonetheless quite sophisticated. Prior to Pauling’s publication of his electronegativity scale, chemists either had to rely upon their own best guesses to determine bond affinities or, if they wanted more precision, compute bond energies for every interaction. Pauling’s scale both standardized and simplified these processes while creating a context where chemists could make predictions of “the energies of bonds for which no experimental data are available.”
Clearly one key piece of utility that the electronegativity scale provided was the ability for chemists to draw conclusions without the need for a lot of computation. For example, based on a molecule’s electronegativity, chemists could roughly deduce the ionic nature of a bond. However, in order to predict the ionic character of a bond, Pauling had first needed to make some assumptions, such as creating an “arbitrarily chosen starting point” to which everything is relative. Even though this approach was not precise, Pauling argued that its usefulness justified the simplification of the approach. And naturally, over time, Pauling’s electronegativity scale became more honed and more specific, an evolution that Pauling also predicted in the fourth paper.
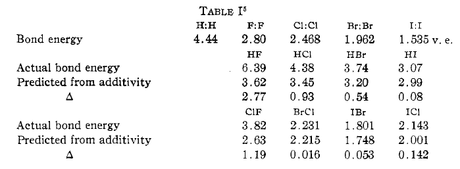
Most of Pauling’s article was devoted to a discussion of how he developed the scale and what kinds of measurements were used in the calculations. To begin, he focused on the qualities of covalent attraction or repulsion in compounds formed by identical elements, such as H:H or Cl:Cl. From there, Pauling used quantum mechanical wave function properties to establish that “energies of normal covalent bonds are additive.” It was from this central theorem that Pauling built out the rest of his conceptual work and also his scale, predicting, for example, bond energies for light atoms and halogens.
Pauling also relied upon others’ helpful calculations in creating his scale, and especially those related to heats of “formation and combustion of gaseous materials” put forth in the International Critical Tables (National Research Council) and elsewhere. The heats of formation were especially useful because they helped to correct for any unknown bond energies. In fact, by using these experimental data for twenty-one different single bond energies, Pauling was able to derive a formula that predicted where a given element should reside on the electronegativity scale. For bonds where data were not available, Pauling used his predictive models to extrapolate approximate energies.
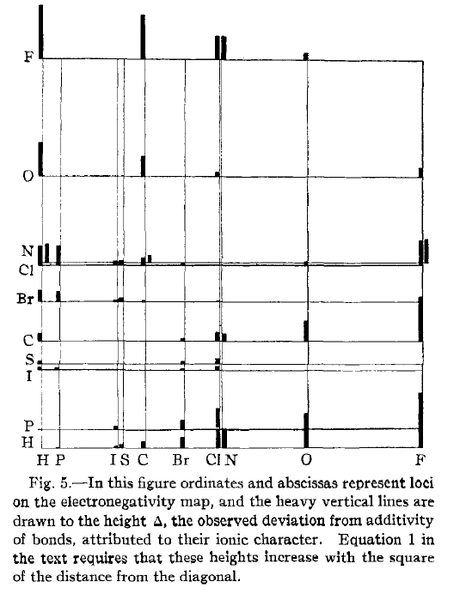
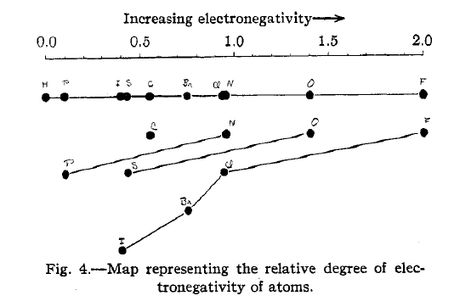
The formula that Pauling derived and published was Δa:b=(χA-χB)2 where χA and χB represent the coordinates of each atom A and B on an electronegativity map (see Fig. 5 above), and Δ represents the degree of electronegativity.
Here, for example, is how Pauling calculated the electronegativity value for oxygen. He began by establishing the heat of formation of water:
2H + O=H2O(g) +9.493 v.e.
Then, based on the fact that the H:O bond is found to have a bond energy of 4.747 v.e., Pauling further calculated that:
H2O2(l)=H2O(l) + ½ O2 + 1.02 v.e.
Pauling next combined this value with the heat of vaporization of H2O2, which is .50 v.e., and found that:
2H + 2O = H2O2(g) +10.99 v.e.
From there, using his original postulate about consistent bond energies, Pauling subtracted 4.75 v.e. for each H:O bond to yield 1.49 v.e. for the O:O bond. However, since Pauling had previously concluded in his previous papers that O:O was a double bond, the actual electronegativity for the oxygen molecule was found to be 3.47. (The present-day electronegativity value for oxygen has been revised to 3.44.)
Even though it relied in part on a collection of assumptions and simplifications, Pauling’s electronegativity scale has been widely used and stands as a lasting component of his legacy. In addition to its ability to approximate values for a wide variety of compounds, the scale was also important for establishing the idea that electronegativity is not a fixed number that never changes. Instead, Pauling understood that an element’s electronegativity value emerges from its bonding relationships. Because of this, calculating absolute values has been difficult, but Pauling’s scale continues to be useful and predictive.
