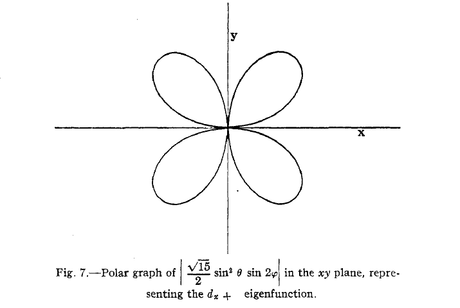
[Part 1 of 7]
Over the span of just two short years beginning in 1931, Linus Pauling published seven decidedly influential papers on the nature of the chemical bond. In the series, which formed the foundation for his 1939 book, The Nature of the Chemical Bond, Pauling introduced many chemists to the burgeoning field of quantum mechanics and demonstrated its applicability to structural chemistry. As a result of this work, Pauling was awarded the 1954 Nobel Prize in chemistry, “for his research into the nature of the chemical bond and its application to the elucidation of the structure of complex substances.” With today’s post, we begin a series that attempts to explore the scientific advancements and ideas put forth in each of the seven papers.
“The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules.” Journal of the American Chemical Society, April 1931.
The first paper in the series was perhaps the most important, in that it set the theoretical foundation for the six papers – some of them more practical than theoretical – yet to come. In it, Pauling sought to lay the groundwork for ideas that he would expand upon in future articles, notably pointing out that “many more results of chemical significance can be obtained from the quantum mechanical equations.” A statement of this sort was necessary because, up until that point, quantum mechanics had mostly been seen as a tool for physicists to use within their discipline. In order to convince chemists of the usefulness of quantum mechanics to their own work, Pauling put forth a conceptual framework consisting of a series of rules as well as guidance on how to apply them to molecular orbitals.
Three of the six rules that Pauling stressed in his paper were already well-known to chemists, but were needed in order to generate “buy-in” for the three additional rules that were new to the field. The most important of these was the fifth rule, which stipulated that when two electrons are in the process of forming a bond, the electron with the larger eigenfunction value will dictate the direction and shape of the bonds that are subsequently created. Pauling argued that this type of electron pairing would result in the most stable bonds possible.
Importantly, Pauling’s rules also combined ideas about wave functions with quantum mechanical thinking in a way that pushed the field forward. Earlier iterations of quantum ideas were not holding up well to certain experimental data. For example, it was known that electrons occupied quanta states, but the observations of the energy associated with these states did not always match what might be predicted by quantum theory. Through his study of both wave theory and quantum mechanics, Pauling recognized that shared bonding energies could explain certain observed bond energies and angles. As such, with his rules, Pauling helped chemists to merge quantum mechanics and wave functions, in the process creating a model that was predictive for all molecules.
In addition to conceptual rules for electron-pair bonding, the first article also helped to establish rules regarding the splitting or breaking of molecular orbitals, a concept that was completely new. The traditionally held belief was that orbitals were firmly set, but Pauling believed that if this were not true, then a whole new set of tantalizing possibilities was on the table. (Indeed, Pauling’s idea that orbitals could be split or broken formed the germ of his later work on the theory of resonance, which proved hugely influential.)
In this section of his paper, Pauling’s main focus was the s and p orbitals. Prior to his article, these orbitals were thought to occupy discrete quantum states that could not be broken. However, based on his earlier assertions that combined wave functions and quantum mechanics, Pauling argued that quantized states could, in fact, sometimes be broken. In subsequent writings, Pauling would conclude that the hybridization of orbitals allowed for the breaking of quantized states, but in this earlier phase of his thinking, he instead put forth that the orbitals were simply broken.
This was, of course, a big theoretical leap, but throughout his series of seven papers, Pauling often chose to base theory on informed guesses, even if he did not always have a precise understanding of how the rules worked. In this particular instance, Pauling used his fifth rule to rationalize the idea of breaking orbitals, positing that, when bonding, the stronger of two electrons would force the weaker to overlap and ultimately create new bond angles.
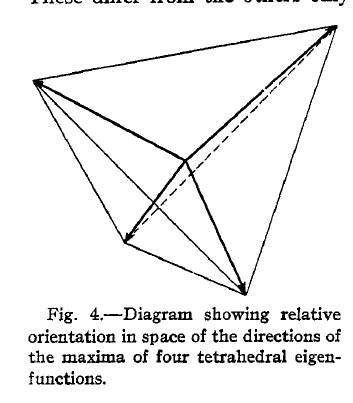
Though he did not yet fully understand all the minutiae of how it could be possible for orbitals to break, Pauling did know that the rules governing chemistry at the time were not sufficient, since they were unable to explain why molecules were sometimes observed to be more stable than predicted. By leaning on the notion that orbitals could break, Pauling was able to devise a set of rules that overlapped almost perfectly with the experimental data. In his paper, Pauling spoke particularly of the tetrahedral carbon atom,
in which only the s and p eigenfunctions contribute to bond formation and in which the quantization in the polar coordinates is broken can form one, two, three, or four equivalent bonds, which are directed towards the corners of a regular tetrahedron.
The idea of a tetrahedral angle is well-known within structural chemistry today, but it was a novel concept in 1931. As such, with his first paper, Pauling was not only proposing that orbitals could do things previously unheard of (i.e. break), but that they also formed angles that were completely new. Pauling knew that these ideas were revolutionary and devoted a significant component of his article to describing the tetrahedral angle in detail.
The s and p orbitals that Pauling addressed were important to many chemists because they formed the building blocks of carbon atoms. Analysis of larger orbitals however, such as the d orbitals, was often kept separate from discussions of the s and p orbitals, because the chemistry of the time lacked a unified law that could apply to both smaller and larger orbitals alike.
Pauling combated this by explaining that his six rules were constant, and that they could be applied to all scenarios, not just the s and p orbitals. From there he reasoned that, while more complicated molecules might open up additional possibilities for bond angles, the proclivity for bonds to form tetrahedral angles still applied. Pauling further argued that this idea was supported by the experimental data. In the case of cobalt for example, Pauling noted that both the predicted and observed angles are six equivalent sp3d2 bonds, and that because of his fifth rule, the bonds are pulled towards the corners of the octahedron that is formed, rather than the center.
As if that weren’t enough, Pauling also addressed magnetization in his paper, a new concept that had long fascinated him. Even during his years as an undergraduate student at Oregon Agriculture College, Pauling was deeply interested in understanding how it was possible that certain compounds were magnetic, while others were not. What, Pauling asked himself, was causing the difference?
In the first paper, Pauling does not quite offer an answer, but he did lay out a series of observations that would lead to new insights on electronegativity, the subject of his fourth paper in the series. In paper one, Pauling specifically contended that, since unpaired electrons were fundamental to magnetic compounds, a model of the bond types that comprise a given molecule could be built using data on the magnetic properties of the molecule. Offering the transition group elements as an example, Pauling pointed out that, without exception, they pair with CN– to form electron-pair bonds; with F– to form ionic bonds; and with H2O to form ion-dipole bonds.
Later in life, Pauling reflected that his first paper on the nature of the chemical bond was “the best work I’ve ever done,” and indeed it is difficult to overstate the importance of the publication. In a single article, Pauling was able to put forth crucial new ideas on bonding in both simple and complex molecular structures using a standardized set of rules. The paper also began the process of applying the new quantum mechanics to help explain the structure of molecules in ways that better supported experimental observations. And while Pauling was later criticized by some for the assumptions that he had made, the value of the paper increasingly shone through as chemists came to understand the practical utility of the work to their own research.
