[An examination of General Chemistry, published by Linus Pauling seventy years ago. This is part 3 of 7.]
The first edition of Linus Pauling’s General Chemistry textbook was published by W.H. Freeman and Co. in August 1947, and almost immediately the comment cards poured in. The majority of the book’s readers praised Pauling’s refreshingly modern approach to the principles of chemistry. They considered his focus on modern chemical principles, with only brief and necessary digressions into historical background, a welcome innovation in textbook design.
Many also were also impressed by Pauling’s clear and direct approach to his subject matter, with one reviewer commenting that
[Pauling’s book] is written in a way which should appeal to the imaginations of those who happen to possess them, which is perhaps as important as anything that can be done.
Reviewers were likewise nearly unanimous in their enthusiasm for Roger Hayward’s skillful illustrations, pointing out the degree to which his depictions were a truly extraordinary asset, especially for concepts known to be sources of difficulty for students.

That is not to suggest, however, that the first edition was uniformly accepted as flawless. Indeed, over half of Pauling’s reviewers declared the book to be too advanced for it’s announced audience: first year students. Moreover, many also noted that the book actually discouraged all but their most determined introductory pupils from moving forward because of the difficulties that they encountered with some of the fundamental principles that Pauling laid out.
Many of the professors who found the text to be too challenging believed that Pauling had mistakenly used Caltech as the standard by which to measure all incoming college freshman. But while their opinion that Pauling had miscalculated in this regard was fairly consistent, the collective did not provide a consensus on who might be an appropriate target audience for the book; reviewers’ suggestions ranged from advanced freshmen to pre-professional students.
In fact, Pauling did have Caltech freshmen in mind when he wrote the book, and very intentionally so. When he embarked upon the project, he made clear that his primary ambition was to develop a text that would prove useful to students who shared his own early enthusiasm for chemistry and who were prepared to devote their academic careers – and, ideally, their professional careers – to the study of chemistry. In the eyes of many though, this approach was not appropriate to other institutions of higher learning and the question of ideal audience remained a point of contention for the entire life of the book.

Several professors who argued in support of the text tended to feel that its advanced nature was actually the best possible asset that could be provided to specific cohorts of students: in particular, students blessed with sufficient high school experience and/or interest, as well as students who made up for their lack of experience with enthusiasm and perseverance. A few years after Pauling first published his book, a California professor commented that, in his higher-level class, students using General Chemistry applied essential material more effectively and achieved higher rates of success in upper-division chemistry courses overall.
Other positive reviewers focused more intently on Pauling’s technique and powers of description. Scripps professor Norris Rakestraw called Pauling’s review of molecular structure “one of the best approaches to an understanding of general chemistry” and also agreed with others who claimed that the book had the potential to propel students to a more rigorous level. If perhaps not ideally suited for freshmen, Rakestraw believed that General Chemistry was certainly perfect for a refresher course.
Several others followed suit. A.L. Rathmer suggested that Pauling’s “unorthodox and unconventional” treatment was valuable to teachers and other researchers in the field. A reviewer from Northwestern University phrased a similar sentiment in a decidedly different way:
Any instructor who fails to read this text should be fired – and any instructor who tries to use it with freshman should also be fired.
A smaller group of professors offering mixed reviews pointed out that Pauling’s own research interests – particularly his biochemical interests – seemed to dominate the text. A few went so far as to accuse Pauling of using his book as a platform to advance his own scientific theories. Pauling, who was generally open to feedback, did not respond to these comments except to disclaim them in fragmented notes scribbled in the margins of letters and review cards.

One oft-issued request that did receive a response from Pauling was that he release an answer key to the book’s exercises. He worked on this key during the summer of 1947 while he was working as a visiting professor at Oxford University, and he enlisted his son, Peter, to work through the problems alongside him, verifying answers and identifying problematic practice questions.
Reacting to a different set of complaints, Bill Freeman suggested that the publishing house also compile and release a laboratory manual that was specifically designed to accompany the text. Settling on a length of 290 pages with 80 Hayward illustrations, Freeman worked with Pauling to select Harper Frantz, a lecturer at Pasadena City College, and Lloyd Malm, a University of Utah chemistry professor, as co-authors. Frantz and Malm in turn developed experiments that were based on and that further amplified the principles described in General Chemistry.
Pauling was not only unconventional in his approach to subject matter, but also in how he used his terminology. Two prominent examples of this tendency were his representation of Avogadro’s number and his use of the term “molality” in the place of “molarity.”
Avogadro’s number defines the units in one mole of a solution and is typically set at 0.6022 x 1023. Pauling, however, chose to write the number as 0.6024 x 1024. Even when several colleagues urged him to stick to generally accepted convention, Pauling insisted on representing the number as 0.6024 x 1024 and continued to do so throughout all three editions of General Chemistry. In defending his point of view, Pauling offered this explanation in a footnote:
There is great convenience in learning Avogadro’s number as 0.6024 x 1024. An important use of this number involves the conversion of the volume of a gram-atom of an element into the volume per atom. The first volume is expressed in cm3, and the second in Å3. The relation between cm3 and Å3 involves the factor of 1024 : 1 cm3 = 1024 Å3. Accordingly, in case that Avogadro’s number has been taken as 0.6024×1024, there is no trouble whatever in deciding on the position of the decimal point.
In other words, by simplifying Avogadro’s number in an unorthodox way, Pauling was trying to make the concept easier to learn for students.
He defended his choice to use “molality” in a similar fashion, referencing A.A. Noyes, Ernest Swift, and W.C. Bray as among those who used “molality” to refer to moles per liter of solution. Pauling incorrectly believed that the term would eventually prevail within the discipline and therefore felt that students would be well-advised to familiarize themselves with it. Eventually he conceded that his was not destined to be the conventional wisdom and he changed “molal” to “molar” in the second edition.
Indeed, throughout the lifespan of General Chemistry, Pauling trusted that students using the book would come equipped with a firm grasp on the language of chemistry. He did, however, agree to provide more definitions in the second edition, particularly for less common or more advanced terms.

Ava Helen and Linus Pauling, 1948.
While there was widespread disagreement about the appropriateness of using Pauling’s book in freshman classes, very few reviewers disputed General Chemistry‘s strength in content. One colleague, J.S. Coles, who later went on to author a textbook of his own, published a review of Pauling’s first edition and followed it up with a letter in which he emphasized that
Even if every suggestion or criticism [contained within the review] were completely ignored, I would continue to believe [General Chemistry] far ahead of any other text in the field and would continue to use it in any of my courses wherein I thought it to be appropriate.
Many professors who did not adopt the text for their courses admitted to keeping a few copies on hand for themselves and, on occasion, for their advanced students. More still reconsidered their initial rejection of the book when the Frantz-Malm laboratory manual came out in 1949. Of the overall response to General Chemistry, Bill Freeman wrote, “Oh we will slip from grace now and then – I hope it will be because we are trying to improve on the conventional.”
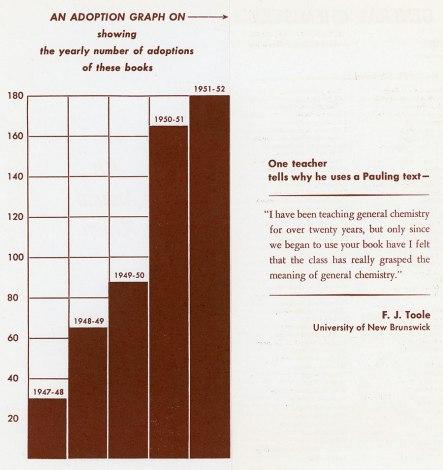
What is beyond doubt is that General Chemistry was wildly successful, even if it didn’t always reach its intended audience, and the royalties that Pauling received provided him with a new level of financial comfort. While most of the windfall was used to support Pauling’s ambitious travel schedule, he did choose to invest in at least one comfort item: an outdoor pool at his Pasadena home.
Affectionately dubbed by his children as “the pool that General Chemistry built,” the space quickly became a gathering spot for some of Pauling’s luckier graduate students, and evolved from there into a location where students could engage Pauling in lively conversations and solicit advice. Not unlike Pauling’s book, these conversations, on any number of occasions, led to insights that shifted the entire course of a student’s professional trajectory.
Advertisements &b; &b;