
[Ed Note: Today we begin a lengthy examination of Linus Pauling’s milestone textbook, General Chemistry, which was published seventy years ago. This is part 1 of 6.]
Linus Pauling’s first college textbook, General Chemistry, revolutionized science textbook publishing and changed how professors, students, and authors approached introductory texts. The first edition was published in 1947 by a fledgling independent press, W.H. Freeman and Company, that enjoyed its first taste of success as a result of Pauling’s book. And what a success it was! General Chemistry was on the market for over twenty years, was translated into more than ten languages, and was adopted at almost 200 universities in the United States alone. Over the next several weeks, we will endeavor to tell the story of how this book came to be and the significant impact that it ultimately made.
Several years before Pauling set out to write a book himself, he noted that the science texts in current use – particularly freshman and introductory texts – often failed to keep up with new and updated theories. This concerned Pauling as he firmly believed that introductory texts were an important foundation to a scientific education.
He likewise worried that authors of such texts often attempted to cram too much information into their pages and in the process lessened the student’s opportunities to gain practical experience from their education. In addition, Pauling felt that authors of the day tended to present their subjects chaotically and too often failed to distinguish guiding principles from constituent components. Even when an author did identify distinguishing principles, Pauling complained that their bias further inhibited students.
Clearly this situation could be improved upon, and in his initial notes on the topic, Pauling began to sketch out a vision for what would later come to pass, recording a variety of observations like the following:
I believe that a book would be valuable to young students which gave them concrete pictures of molecules as we now picture them. Ionic substances could well be described as containing spherical [sic] given by ‘crystal radii,’ the electrons staying mostly within.
As he surveyed the pedagogical landscape, Pauling identified a particular need for improvement in instruction on theories of atomic structure as well as ideas emerging from quantum mechanics, statistical mechanics, and thermodynamics. Accordingly, he began preparing lessons and supplementary materials on these topics for use in his own teaching. These materials, which eventually took on the form of a booklet, touched upon new theories while also providing concise explanations and discussions of the practical applications of various older theories. Pauling updated this booklet frequently, using it to supplement Joel Henry Hildebrand’s Principles of Chemistry, the textbook of choice for many introductory-level chemistry classes, including those taught at Caltech.
As time moved forward, Pauling became more serious about reformatting and publishing a version of his classroom booklet, which mostly consisted of a semi-formal collection of notes. As he developed his publication plan, Pauling drew up an outline of the subjects that would want to discuss in his text. He also jotted down thoughts on general formatting as well as broad introductory remarks on the importance, history, and daily application of chemistry. Having thrived as a lecturer for over a decade already, Pauling felt that he had far more to offer to students than a rote recitation of past discoveries, results and publications. Indeed, his ultimate ambition was to re-imagine the very foundations of chemistry education.

Pauling eventually had his revised booklet, which he titled Elementary Chemistry: The Facts and Basics of Chemistry and their Significance in Modern Life, lithographed for the purpose of aesthetics and ease. As he later remarked to publisher Bill Freeman, he did not consider this collection, even after he had it lithographed, to be a separate draft. On the contrary, this version of what would become a major textbook was to be regarded as a snapshot of a stage in Pauling’s writing process.
Pauling didn’t formally announce his plans to publish a textbook until 1941, by which time he had generated a more organized draft of thirteen mimeographed chapters. He changed the title of his manuscript to General Chemistry: A First-year Course to Follow a Year of High School Chemistry, and by the early months of 1942 he had a group of ten publishers competing for the opportunity to publish his book.
Choosing which publishing offer to accept proved to be a difficult process for Pauling. By 1942, Pauling had emerged as a star within the world of science and several publishers recognized full-well the likely benefit of having his name associated with their company. For his part, Pauling strove to find a publisher who recognized the value of the book itself, regardless of the name attached to it.
John Wiley & Sons was the first company to approach Pauling about his manuscript but the relationship quickly soured, partly because Pauling had become extremely busy. Burdened by a great many other duties, Pauling did not appreciate Wiley’s stipulation that he wait until they had “thoroughly examined the manuscript” before allowing him to send it anywhere else. The final straw came about when Wiley expressed skepticism that Pauling would finish his text in a timely manner; annoyed, Pauling withdrew the manuscript from their consideration.
W.B. Saunders Company approached Pauling next and expressed such a genuine and deep interest in his work that Pauling began negotiating with them shortly after they had made their initial pitch. Saunders had substantial experience publishing scientific texts and, unlike Wiley & Co., believed so completely in the Pauling book’s potential to succeed that they proposed a royalty rate of 15% for each retail sale. (J.C. Stacey Inc., another company in the running at the time, learned what Saunders was proposing and advised Pauling to accept the offer, as 15% far exceeded the standard royalty rate being tendered to authors at that time.) Saunders also offered to send Pauling’s preliminary draft to a chemistry professor for initial feedback and to finance a graduate student to assist with the detail work.
Encouraged by the host of publishers clamoring to publish his work, Pauling continued to revise his manuscript. This steady rhythm was interrupted when the United States entered World War II, a point at which Pauling quickly realized that his government-funded war projects were going to require his full attention. Discouraged from writing, but recognizing the importance of what he was doing, he sent out a copy letter to all publishers interested in his manuscript. In it, he stated that the present circumstances were such that he was unlikely to make much progress on his book. When the war concluded in 1945, many of these publishers inquired again, but by then Pauling had made his decision.
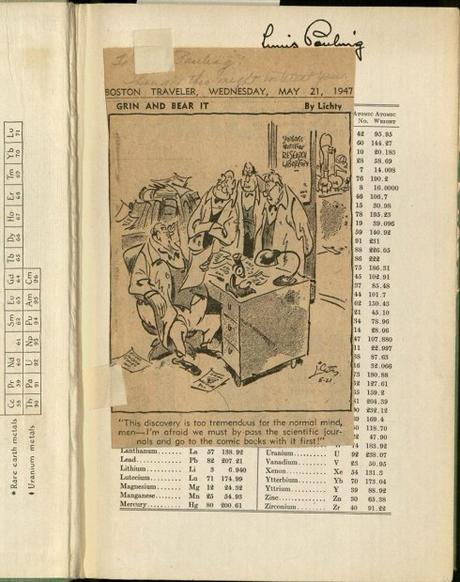
Cartoon tipped into Pauling’s first edition, first printing of General Chemistry, 1947.
William Freeman, a representative of the college department at MacMillan Publishers, had approached Pauling in 1941 to express MacMillan’s interest in Pauling’s manuscript. A strong contender from the beginning, MacMillan had enticed Pauling with their experience as a mainstream textbook publisher. The company’s associate editor also promised Pauling the best editing services and attention around.
In the end however, it was Bill Freeman who won Pauling over. Since Freeman worked at MacMillan’s San Francisco branch, he offered to meet with Pauling in Pasadena to discuss, in person, the means by which they might partner to navigate the publishing world and protect Pauling’s rights as an author. Pauling was concerned that Macmillan, though a major player in the textbook industry, hadn’t published many scientific volumes. Freeman replied candidly, pointing out that this was actually a good thing because it meant that Pauling wouldn’t have to compete for marketing resources within the company.
Freeman also assured Pauling that, although a high royalty might look good in the short-term, a lower royalty, such as the one that MacMillan was offering, would allow the company to market the book at a lower cost. Doing so, Freeman argued, would ensure a higher volume of sales for Pauling’s text and, consequently, a more widespread adoption. Pauling was impressed. After meeting with Freeman, he returned Saunders’ contract completely blank.
When the U.S. entered the war, Pauling sent MacMillan the same letter that he had sent to everyone else, detailing the time conflicts that he was confronting with his scientific war work and announcing that the book project was moving to the back burner. But as the war years went by, Pauling and Freeman stayed in touch, and the relationship that the two men developed during this period made all the difference.
When Freeman decided to strike out on his own as an independent textbook publisher with a focus on science, he recruited Pauling to edit a series of chemistry books that he planned to publish over the next decade. In turn, Pauling entrusted his own coveted manuscript to Freeman as the first book to be released in this series and, ultimately, the first text that W.H. Freeman and Company would ever publish.
Advertisements