[A guest post expanding on Pauling's idea of a science fiction novel. Post authored by the blog's East Coast Bureau Chief, Dr. John Leavitt, Nerac, Inc., Tolland, CT.]

Pauling lecturing with the “fish model” (foreground) that he used to demonstrate chirality, ca. 1960s.
In basic chemistry we have something called “chirality” which refers to a molecule with two possible non-superimposable configurations. One way to picture this is to look at your hands and place one on top of the other (not palm to palm) – your left and right hands are essentially the same shape but their shape is reversed. At the molecular level we can use one of the main building blocks of all proteins and all life – the amino acid alanine, depicted in the image below – to examine handedness.
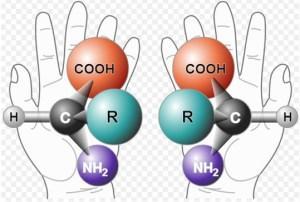
The diagram shows the arrangement of atoms of two alanine molecules, both of which exist in nature, arranged so that they are mirror images. They are the same molecules but if you turn the one on the right around so that it is facing in the same direction as the one on the left, the R (a single carbon atom in alanine with three bonded hydrogen atoms) on this alanine molecule faces toward the palm of the hand and the COOH moiety (a carboxyl group) and the NH2 moiety (an amino group) face outward away from the palm.
No matter how you rotate the alanine on the right, you can’t get the three moieties attached to the central carbon to line up in the same position as the alanine on the left. Likewise, you can’t get those hands to super-impose each other no matter how much you twist and turn them. So the alanine on the left is called L-alanine (levo- for the direction the molecule rotates photons) and the alanine on the right is called D-alanine (dextro- for the direction the molecule rotates photons). They are called “enantiomers,” or chiral forms, of alanine, and both exist in nature with identical chemical properties except for the way that they rotate polarized light.
There are twenty natural amino acids comprising the building blocks of all proteins. Of these twenty, only glycine is symmetrical around a central carbon atom and therefore glycine has no enantiomers. The other nineteen can exist in the L- and D-conformation.
Funny thing though, only the L-enantiomer is used to make proteins by the protein synthetic machinery of all life-forms, from single-cell organisms up to humans. It’s quite easy to understand why one enantiomer is used in life over random use of either enantiomer. In explaining this, note the pictures below, which show the three-dimensional globular structure of human beta-actin on the left and, on the right, the architectural arrangement of this actin in the cytoplasm of a cell.
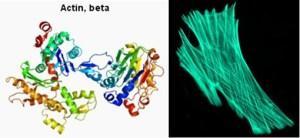
The protein composed of 374 amino acids has an intricate folding pattern with coils which would not be possible if both amino acid enantiomers for the nineteen amino acids were randomly incorporated into the protein. This three-dimensional structure has to be preserved in order for actin to perform its dynamic architectural function inside living cells, as shown in the picture on the right. The coils are possible because the amino acids are all L-amino acids and glycine is neutral; otherwise the protein would behave like a wet noodle. The precise structure of the actin protein determines its function, which has been preserved and conserved since the beginning of all eukaryotic life-forms (that is, cells with a cytoplasm and a nucleus). Understanding the atomic forces that fold proteins in a unique shape is part of the reason why Linus Pauling received the Nobel Prize for Chemistry in 1954.
Aside from those who closely follow this blog, it is not well known that Linus Pauling was an avid reader of science fiction. In a 1992 interview with biographer Thomas Hager, he described his motivation to write a science fiction novel. The story line was to be the discovery of a human-like race from another planet that had evolved to use only D-amino acids (D-humans) rather than the L-isoform (L-humans). He explained that he never got around to writing this novel because the real science he was doing took all of his time.
If our L-humans met up with those D-humans, what consequences would there be? Well, what we would see in D-humans are people virtually indistinguishable from ourselves – barring, of course, the possibility that these extraterrestrials evolved out of some unearthly environmental niche. However, no mating, blood, or tissue sharing would be possible between these two races.
To explain this, consider the experience you have had when you accidently put your hand in the wrong glove. As you know, this doesn’t work well. All protein interactions and reactions catalyzed by enzymes require a direct fit to work. Substrates of enzymes have to fit precisely into the catalytic active site of the enzyme, like your hand fitting into the correct glove. Since L-humans have a different chirality from D-humans, nothing would fit or be transferrable, because of asymmetric incompatibility between L- and D- macromolecules. Even the food on our planet would not likely be nutritious for D-humans because all living things on Earth are L-organisms. In D-lifeforms, the actin coils would coil in the opposite direction and the DNA double helix would have to spiral in the opposite direction as well; otherwise the analogous D-proteins would not bind or fit on the chromosomal DNA.

It seems reasonable that D-humans might be found on other planets if you consider how life got started. By a quirk of nature on Earth, L-amino acids got a head start and self-assembled into peptides (small proteins) when this essential process for life as we know it got started. The assembly of only one enantiomer isoform into a peptide may have been favored thermodynamically over co-random assembly of L- and D-isoforms. This essential process evolved into a well-organized, membrane-protected and energy-driven protein synthetic machinery in single cell organisms like bacteria. Today, humans have essentially the same protein synthetic machinery that evolved in primordial bacteria and all life-forms on Earth have the same genetic code.
There are two essential enzymes that work together to catalyze protein synthesis in all living cells. One enzyme, called aminocacyl-tRNA synthetase, binds the amino acid to a transfer RNA molecule (there is one of these enzymes and a specific tRNA for each of the twenty amino acids). The second enzyme, peptidyl transferase, catalyzes the formation of a peptide bond linking two amino acids at the start of a chain and does this over and over again until the full length protein is synthesized and folded into its functional conformation. These two essential enzymes do not recognize the D-isoforms of the nineteen asymmetric amino acids. Thus, our chiral L-specificity has been preserved since the beginning of life.
I can’t think of any reason why the D-amino acids would not support life, but it has to be one isoform or the other, not both. Apparently Pauling felt the same way. Should it ever come to pass, D-humans will be interesting to meet and they will be equally interested to meet us, hopefully without mutual disappointment.
