In this article, please revisit an article written two years ago titled, "The Calm Before the Storm." This article focused on the patent cliff that was looming in the pharmaceutical industry, that was later picked up by the New York Times and several other bloggers! Subsequent articles were written about big pharma company's revenue streams, and the pros and cons of of their later stage pipelines. Other articles have also attempted to identify smaller biotechs with the potential to reap big rewards, because these biotechs are possible acquisition candidates by the big pharma companies that need to fill their coffers with new, multi-billion dollar drugs - the so called "blockbusters."
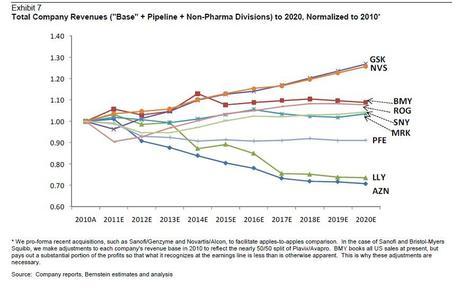
Forbes has an informative article that projects three different revenue scenarios in in the pharmaceutical industry. Forbes's last figure (below) shows what companies will best weather the storm by combining "total company revenues."
In the 'Storm' article, it is noted that pharma growth rates should be growing less or starting to stall into 2014 due to the patent cliffs (see Table 1). A few examples of slow growing revenue streams are (2010 -2011): Pfizer $67.1B to $67.4B; Merck $46B to $48B, JNJ $62B to $62B, and AMGN $15.1 to $15.5. The industry is still ripe for consolidation based upon forward earnings, and the numbers of blockbusters will be dwindling. The industry is taking on more risk in therapy areas that are unmet medical needs, but have fewer patients to treat – thus higher prices will ensue. Monoclonal antibodies (mAb) for cancer and other 'biologics' fit this pricey niche area.
Company Drug Indication Sales in $B (2009) Patent Expiration
Pfizer/Eisai Aricept Alzheimer’s 1 2010
Merck
Cozaar
Hypertension
3.6
2010
Pfizer
Lipitor
Cholesterol
12.5
2011
JnJ
Levaquin
Antibiotic
1.5
2011
Sanofi/BMS
Plavix
Anticoagulant
9.3
2011
AstraZeneca Seroquel Antipsychotic 4.9 2011
Lilly
Zyprexa
Antipsychotic
4.9
2011
Merck Singulair Asthma 4.7 2012
Forest Labs/Lundbenk Lexapro Antidepressant 2.3 2012
AstraZeneca Symbicort Asthma 2.3 2012
Novartis Diovan Hypertension 2.9 2012
49.9
There are two ways that pharma companies can grow – acquisitions and internal innovation. In member chat, we have been focusing on those smaller companies that have internal innovation and the possibility of being acquired.
What are big pharma companies looking for in terms of possible acquisition targets?
- Companies that are in late stage clinical trials.
- Companies with blockbuster drug potential, which is one with a potential sales of $1B/year.
- Companies with very high chances of success in the Phase III trials.
- Companies that own all world-wide rights to their product and have long term patent protection.
This article updated the approvals and later stage pipeline candidates on three companies, Bristol-Myers Squibb (BMY), Merck (MRK), and Pfizer (PFE). Future articles will follow up on other big pharma companies.

BMY has had some nice hits over the past year to help offset the loss of Plavixx.
-
Apixaban - also known as Eliquis (approved in the EU, not in the US yet), is co-marketed with Pfizer. BMY is is looking to apixaban to make up some of the clopidogrel revenue. Apixaban is a direct factor Xa inhibitor (like JNJ/Bayer’s rivaroxaban), but BMY took a risk and went for 2X/day dosing to reduce any potential adverse events (AEs). Well, they won against JNJ, and should steal most of the market. Apixaban reduced the risk of stroke in patients with a heartbeat irregularity called atrial fibrillation by 21% and lowered by 31% the incidence of bleeding, a serious side effect of warfarin. The twice-a-day pill also cut patients' risk of death by 11% over a follow-up of nearly two years. It is projected to bring in $4B by 2015.
-
Ipilimumab – also called Yervoy was approved in March 2011, is projected to bring in >$1B for the treatment of melanoma. Melanoma is one of the most lethal cancers, so when researchers pulled the veil back on data showing that the therapy significantly improved one- and two-year survival rates, chances of an approval shot up.
-
Dapagliflozin – Dapagliflozin inhibits subtype 2 of the sodium-glucose transport proteins (SGLT2), which is responsible for at least 90% of the glucose reabsorption in the kidney. Blocking this transporter causes blood glucose to be eliminated through the urine. The FDA gave a CRL, but the EU approved the drug a few weeks ago. It will be interesting to see if they can get this approved in the US. Projected sales are >$1B.
-
Daclatasvir (NS5A inhibitor) - HCV (hepatitis C) remains one of the largest unmet medical needs and probably the hottest area in pharma/biotech. Gilead (GILD) purchased Pharmasset (VRUS) for $11B (YIKES) (which was right in BMY's backyard of Princeton). Daclatasvir works well, and when in combination with other HCV drugs (cocktail solution), the disease is almost wiped out. BMY purchased Inhibitex for $2.5B to bolster their pipeline, and now MRK, BMY, GILD and others need to mix and match their cocktails to see who will win out on this market. Think of it like the cholestrol market of yesteryear, with Lipitor, Zocor and Crestor all competing in the same space.
-
The company has a wide range of things in Phase 2, and some look very attractive IF they work, and AEs remain manageable. A few I like are: Notch inhibitor for cancer, LPA1 antagonist for IPF, GPR119 Agonists for diabeties and obesity, and IKur antagonists for atrial fibrillation.

- MK-4305 is an orexin receptor antagonist designed to inhibit the binding of the neuropeptide orexin to its receptor. MK-4305 is being evaluated for the treatment of insomnia. Why do we need more sleep drugs after Ambien and Lunesta? Well, some who take Ambien/Lunesta complain of 'hangover' feelings, and can be addictive due to their depressing the central-nervous system. Orexin receptor antagonist are supposed to temporarily switch off the brain's arousal symptom but avoid the depression of the central nervous system. Merck plans to file for the NDA (new drug application) later in 2012, and if approved, could generate $500M in annual sales.
- Anacetrapib (MK-0859) is an investigational oral inhibitor of cholesteryl ester transfer protein (CETP), a plasma protein that plays a role in the cholesterol transport pathway. Anacetrapib is being evaluated for the treatment of atherosclerosis. Pfizer had one of these that was in the middle of an enormous clinical trial, and failed due to a transient increase in blood pressure. Merck forged ahead with theirs, and time will tell if they will bear the fruits of their labor. Early data show that the drug spurred a 138% increase in good cholesterol and a 40% drop in bad cholesterol among patients taking statins. Data are due in 2015. This could be the next Lipitor. Roche/Jappan Tobacco also have a drug in this class in trial, so it will be a race to the finish. Projected sales are >$10B – that's a big market.
- Preladenant (MK-3814) is an investigational adenosine 2A (A2A) receptor antagonist. Preladenant is being evaluated for the treatment of Parkinson's disease. At CHI's Fourth Annual GPCR-Based Drug Discovery Meeting in Boston, MA (2009) that 5- and 25-mg doses improved symptoms as an adjunct to L-dopa. If this works, it may not be a huge selling drug, but anything that can help Parkinson's patients is a good thing. Projected sales are <$1B.
- Odanacatib (MK-0822) is an investigational inhibitor of the enzyme cathepsin K. Odanacatib is being evaluated for the treatment of osteoporosis. It is attempting to replace Fosemaxx. Odanacatib showed an increase in spine and hip bone mineral density (BMD) after four years of follow-up, suggesting that odanacatib use leads to increased bone strength.
- VICTRELIS is a prescription medicine used with the medicines peginterferon alfa and ribavirin (peg/riba) to treat chronic (long-lasting) hepatitis C genotype 1 infection in adults with stable liver problems who have not been treated before or who have failed previous treatment. This drug was approved last year (~$300M in annual sales), but it is not as good as Vertex's Incivek. Unfortunately, there is a lot of competition in this space.
- Ezetimibe + atorvastatin (MK-0653C) is an investigational medicine containing ezetimibe (Zetia) and atorvastatin (Lipitor) for the treatment of dyslipidemia. Milking the cholesterol franchise for all its worth! No comments here, as it is a me too drug, and the FDA just rejected it. Read about it here.
- The company has a wide range of things in Phase 2, and ones to watch are: HPV Vaccine (9 valent) (V503) for prevention of HPV, BRIDION (MK-8616) for reversing muscle relaxation induced by certain muscle relaxants used as part of general anesthesia during surgery, and V419 is an investigational pediatric hexavalent combination vaccine.

Pfizer continues to shed revenue, in 2012 Pfizer will lose exclusivity on Aricept (in the EU and Japan), Geodon ($1B), Revatio ($0.5B), and Detrol IR ($0.9B). 2011 was not friendly either with the loss of Lipitor ($9.6B), in addition PFE lost exclusivity on Aromasin ($0.4B), Caduet ($0.5B), Vfend ($0.8B),and Xalatan ($1.25B). My article here notes that PFE overpaid for Wyeth, and they need to do some work to keep up revenues, as well as shed some weight. The company has been laying off scientists and sales staff over the past few years (close to 50K), and now they need to show something in the pipeline. Another good article was on SeekingAlpha by Analytical Chemist, and I have to agree, PFE is not my favorite play in the pharma marketplace.
- Oncology is a main component of their upcoming revenue streams (potential 2012 launches).
- Bosutinib is quinoline-based, dual-specificity inhibitors of Src and Abl kinases, for the potential oral treatment of chronic myeloid leukaemia.
- Crizotinib is a first-in-class, oral anaplastic lymphoma kinase (ALK) inhibitor currently in Phase III trials for the treatment of advanced non-small cell lung cancer (NSCLC).
- Axitinib is an oral and selective inhibitor of vascular endothelial growth factor (VEGF) receptors 1, 2 and 3 that treats metastatic renal cell carcinoma.
- In the cardiovascular space, Eliquis will be the replacement for Lipitor (see BMY above).
- In the biologics, Taliglucerase alfa (marketed for PLX) for the treatment of Gaucher's Disease, and vaccines have Prevnar/Prevenar 13 for the prevention of pneumococcal disease in adults.
- Immunology has tofacitinib, a JAK inhibitor for the treatment of psoriasis and RA. The PDUFA date is in August, and the fireworks have started already. In ealry 2011, several patients died while on tofacitinib, but it was later noted that only one was due to the drug.
- The rest of PFE's pipeline can be read about here, and a few that stand out to me include anrukinzumab for Ulcerative Colitis, and glucokinase activator PF-04937319 for DM Type 2.
As investors in biotech and pharma, looking for companies that have a healthy pipeline and a history of successfully bringing drugs to the market is the key to investing right. Buying one company, go with one of the best, and BMY and MRK should be good for the years to come. All of these pay a good dividend, and those dividends should remain as time passes. Join us in member chat to see what buy/writes PSW members are buying for our virtual portfolios.
For my latest option strategies for BMY, PFE and MRK, sign in to read this week's Stock World Weekly, or sign up for a trial.

