
[Part 2 of 2]
In the mid-1960s, as he continued to develop his close-packed spheron theory of atomic nuclei, Linus Pauling sought to use the techniques of creative visualization that had served him so well in his past theoretical work. The situation was trickier this time however, as the discipline of nuclear physics had not yet put forth a single agreed-upon understanding of molecular structure on which a visual model might be built. Rather, as Pauling put it:
There are now two ways of considering the structure of a molecule. One of these ways is by application of a highly refined set of ideas about chemical bonds that was largely developed during the period from 1860 to 1900, and was then significantly extended between 1925 and 1935 through consideration of the empirical information about molecular structure in light of basic principles that were introduced by quantum mechanics.
The second way of considering the structure of a molecule is by solving the wave equation for the molecule, describing the state of the various electrons interacting with one another and with the nuclei.
Both methods had been used since quantum mechanics entered the mainstream in the 1920s and began being applied to topics in chemistry. But according to Pauling, very little effort had been made to develop an empirical theory of the structure of atomic nuclei that corresponded nicely with contemporary ideas on chemical structure. The wave equation approach was further complicated by the fact that, by the mid-1960s, reliable quantum mechanical calculations could be carried out for only a small number of molecules.
In Pauling’s estimation, the most useful attempt to date to develop a workable theory was folded into a discussion that conceptualized light nuclei as aggregates of alpha particles – particles that Pauling renamed “helions” and made a centerpiece of his theory. To address the perceived need to move forward with this mode of thinking, Pauling developed his own conceptual framework and promoted it throughout the 1960s.
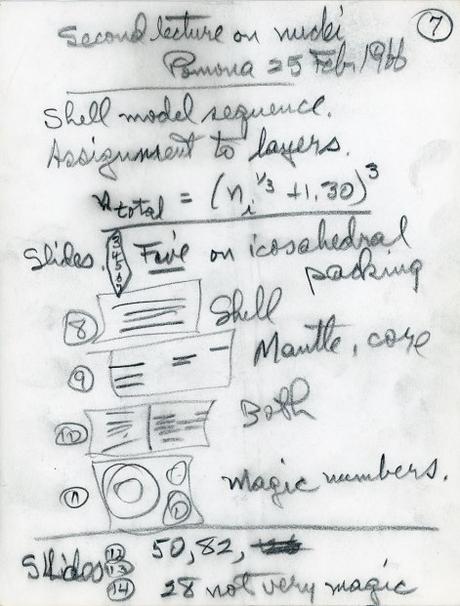
Notes from Pauling’s second Robbins Lecture, February 25, 1966
In 1966 Pauling used his platform as Pomona College’s first Robbins Lecturer to begin spreading a more popular word of this theory — he gave five lectures on the subject in February and March of that year. In 1967 he delivered three more talks on the theory, this time at Princeton University under the auspices of the Plaut Lectureship, an honorific which Pauling was again the first person to occupy. Finally, in 1971, he offered a free public lecture at the University of Colorado, Boulder, promoting his theory as the E.U. Condon Chemical-Physics Lecturer.
Throughout all of this public outreach, Pauling continued to draw up new manuscripts for publication on the topic. In these, he began considering how nuclear wave functions and magnetic-moment values could all be accounted for using polyspheron theory. He also continued to emphasize the compatibility of his ideas with the shell and cluster theories that had gained traction within the discipline.
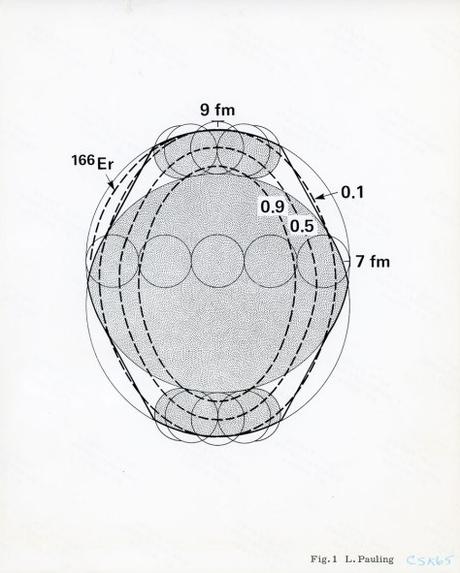
A figure included with Pauling’s 1976 manuscript submission to Physical Review
Pauling’s fight to push polyspheron theory into the physics mainstream continued in 1976, by which time the term “helion” was generally being used by nuclear physicists to refer to a Helium-3 nucleus. This common understanding proved disadvantageous to Pauling, as he had defined the term as referring instead to alpha particles (although, interestingly, Pauling had earlier pointed out that “his” helions were notably present in Helium-3).
But there was also good news for Pauling: by now, scientists using electron scattering techniques to investigate the electromagnetic properties of atomic nuclei with prolate deformation were publishing data that seemed to support his point of view. In particular, these experiments had derived information about the shapes of the deformed nuclei that showed what appeared to be the formation of spherons out of the objects defined by polyspheron theory as “helions” and “tritons.”
Pauling almost immediately pounced on this encouraging data, writing a paper initially titled “Comment on the Shapes of Deformed Nuclei,” and then later more forcefully retitled as “The Predication of the Shapes of Deformed Nuclei by the Polyspheron Theory.” He submitted his manuscript to Physical Review in Spring 1976, arguing that reports from current nuclear physicists had confirmed many of his model’s assumptions and that, accordingly, polyspheron theory merited wider acceptance within the field.
The referee overseeing Pauling’s manuscript asked that he make several revisions prior to publication. One major request was that Pauling reshape some of the terminology that was so specific to his theory, due to its confusing nature with respect to broader accepted nomenclature. The journal also asked that Pauling support his claims with clearer and more substantial calculations.
Pauling responded that the journal’s editors had apparently misunderstood the purpose and the importance of his work, and then appended a more substantive introduction to the beginning of the paper to clarify some of the specifics that had been requested of him. When the journal later asked Pauling to remove the extended introduction he refused and his submission was withdrawn. In 1982, some six years later, a paper with the same title finally made it into print by way of the Proceedings of the National Academy of Sciences, a journal that had historically published most submissions authored by distinguished Academy members, among whom Pauling could certainly count himself.
In 1987 Pauling again attempted to advocate on behalf of his theory, writing a new paper titled “Properties of the High-Spin Superprolate Structure of 15266Dy86 are Explained by the Polyspheron Theory.” This paper once more claimed that his now thirty-year-old theory was being steadily confirmed by contemporary laboratory research.
As in 1976, the 1987 manuscript was also rejected, this time by Physical Review and by Nature alike. Seven years later, right around Pauling’s ninety-third birthday, an article with a far less forthright title did appear, once again in PNAS: “Analysis of a Hyperdeformed Band of 152 66 Dy86 on the Basis of a Structure with Two Revolving Clusters, Each with a Previously Unrecognized Two-Tiered Structure.”
Despite his periodic appeals to the practicality of his theory over the course of several decades, and disregarding his insistence that the theory had been vindicated by its prediction of novel discoveries, Linus Pauling’s ideas on polyspherons were never generally accepted by his peers in either physics or chemistry.
Today the utility of Pauling’s model of the atomic nucleus remains in doubt. What does seem to be clear is that he did not succeed in grasping one of the scientific world’s most elusive holy grails: a unified theory of quantum physics and chemistry.
Advertisements &b; &b;