I'm going to do this one in two parts, in the hope that posting the first half now will force me to find time to write the second part at the weekend. Also, it'll hopefully make for shorter and more readable posts. Enjoy!
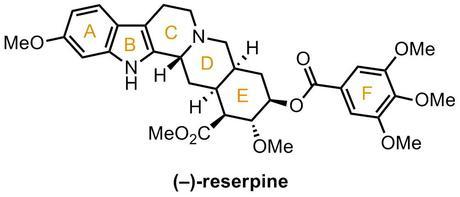
Reserpine is an indole alkaloid isolated in 1952 from the extract of Rauwolfia sepentina or ‘Indian snake root’, a popular plant in traditional Indian medicine used as a sedative and antipyretic, and reportedly taken by Mahatma Ghandi himself. It's also enjoyed some attention from Western doctors as an antihypotensive and antipsychotic, notably being the first ever drug to successfully demonstrate antidepressant properties in a randomized placebo-controlled trial (although it’s rarely used nowadays because of its numerous side effects, which are as varied as they are unpleasant). Its structure was solved in just 3 years (a remarkably short period for the pre-NMR era) and, when it was finally reported in the summer of 1955, Woodward immediately set to work. By the end of 1956, just a year later, he was able to report a detailed series of studies culminating in the landmark first total synthesis of the natural product, again pushing forward the complexity limit at which synthetic chemists could operate. In the years that followed, reserpine became a classic target, worked on by some of the greatest chemists of the past 50 years.[1] The latest one, by Eric Jacobsen, was reported just a month or so ago (Org. Lett., 2013, 3, 706). Although Woodward’s synthesis of this target, supposedly his personal favorite of all those he masterminded, has been discussed in just about every book to be written on the history of total synthesis, I can’t resist the temptation of writing my own summary of it any longer, so here goes.
Woodward’s synthesis began with a reaction that he had been fascinated by since the very beginning of his studies in chemistry: the diene synthesis, or as it’s now known, the Diels-Alder reaction. At the age of just 25 he published a paper on its mechanism in JACS (as the sole author), and he’d also recently applied it successfully to the arena of steroid chemistry where it had been the first step of his successful 1952 formal synthesis of cortisone and cholesterol. Here, benzoquinone was used as the dienophile, and freshly prepared vinylacetic acid as the diene, and the reaction was conducted on grand scale. From the full paper (emphasis mine):
“The condensation of malonic acid with acrolein was carried out in three identical separate batches. In each, 2000 g of malonic acid were added to 4.5 l. of pyridine (technical, containing 0.5 per cent of water) with vigorous stirring. After about 30 min the major part of the malonic acid had dissolved. The mixture was then cooled to 10º in an ice-cold bath, and 1333 g of acrolein were added at such a rate that the temperature did not exceed 12º. The addition was complete in about 90 min; stirring was then continued for 3 hr at 0º and subsequently for 35-40º. (The reaction mixture is very viscous at 0ºC and becomes fairly mobile at 35-40º). At the end of the stirring period, the three batches were combined and slowly added to 13 l. of a 50 per cent (v/v) aqueous solution of sulfuric acid at –5º, with stirring, and at such a rate that the temperature did not exceed 15-20º. The mixture, which was acidic to Congo, was filtered through celite. The clear yellow filtrate was extracted six times with ether (three times with 7 gal and three times with 3 gal each). The ether extracts were twice washed with water, dried over anhydrous sodium sulfate, and concentrated to 3 gal. Six gallons of benzene were then added, and the mixture was again concentrated to 3 gal.
Three and one-half kilograms of quinone (Eastman Kodak Practical, recrystallized by Soxhlet extraction with octane; if large crystals of quinone are present, the material should be ground before use), dissolved in 2 gal of benzene, were added to the above benzene solution of vinylacrylic acid. The mixture was heated under reflux for 3 hr, and then filtered hot (c. 45-50º). The filter cake comprised 2.3 kg of gray crude adduct...”[2]
Reading the experimental section of the reserpine full paper absolutely blew my mind the first time I got hold of a copy.[3] The evocative language, the detail (even for tangential compounds), and the lovingly reproduced IR spectrum given for each compound set the highest standard for supporting information. It’s a great shame that modern SI is so dry and minimalist (not to mention often downright obscure).[4]
Although the yield for the Diels-Alder reaction was quite poor (due to irreversible tautomerisation/aromatisation of the initial adduct), over 2 kg of pure adduct were obtained from each run, providing ample material for investigation of subsequent steps. The product of this reaction provided a great starting point for the synthesis; it contained the reserpine E-ring with three out of its five contiguous stereogenic centres set, as well as a carboxyl group placed ‘felicitously’ and ‘a double bond of good augury for the introduction of oxygen atoms at appropriate positions’. Next, differentiation of the two carbonyl groups was required; as Woodward remarks in the paper ‘we were prepared to make use of the opportunity which would be presented by the selective reduction of either carbonyl group’. Fortunately it was found that treatment of the sodium salt of the Diels-Alder adduct with sodium borohydride provided just such an opportunity, accomplishing selective reduction of the carbonyl remote from the carboxylate. Epoxidation with perbenzoic acid occurred at the more electron rich double bond (from the convex face),[5] and this was immediately followed by lactonisation, effected by treatment with acetic anhydride. Interestingly, if the order of these two steps was reversed, no product was obtained in the epoxidation step.[6] Meerwein–Ponndorf–Verley reduction by treatment with aluminum triisoproxide in isopropanol then caused a remarkable sequence of reactions to take place place, including reduction of the remaining ketone, transesterification to give the 5-membered lactone, epoxide opening by the newly liberated allylic alcohol, and finally elimination of water. The resulting crude α,β-unsaturated ester from this sequence was then treated with sodium methoxide to give the conjugate addition product in a remarkably good yield considering what had been achieved.
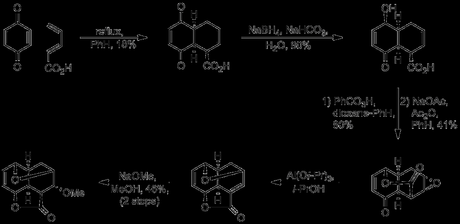
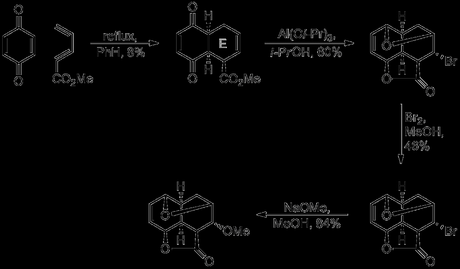
As impressive as the rapid construction of such a stereochemically dense tetracycle with such a simple set of reagents was, Woodward did not yet consider this problem solved to his satisfaction, and published an even shorter route to this same intermediate later in the year. Using the knowledge gained from previous studies, the above six step sequence was shortened to just four steps. The new route began with a modified version of the previous Diels-Alder reaction, employing methyl vinylacrylate in place of vinylacrylic acid. This reaction was unfortunately much lower yielding than that previously used, giving the desired adduct in just 8% yield, although this step was not extensively optimised. In theory, this ester could also be obtained by esterification of the previous Diels-Alder adduct. However, only very low yields were obtained for this step due to aromatisation (under acidic conditions) or side reactions at the olefins (with diazomethane). The ester was now exposed to the same Meerwein–Ponndorf–Verley conditions as before, which reduced both carbonyl groups and also caused lactone formation between the ester and the nearest alcohol. Treatment of this tricyclic lactone with bromine in methanol then effected bromoetherification of the remaining alcohol. Again, both reactions occurred from the convex face of the ring system with essentially perfect stereocontrol. Finally, the bromine atom was displaced by methoxide, with retention of stereochemistry, presumably occurring first by loss of bromide via an E1cB process, followed by conjugate addition of methanol.
From the outset of this campaign, Woodward was said to have regarded the synthesis of reserpine as a problem in stereochemistry, and here is a near-perfect solution that establishes, in just four steps, all five contiguous sterocentres around the E-ring. Despite some rather low yields, tens of grams of the required tetracyclic intermediate could still be obtained using this chemistry.
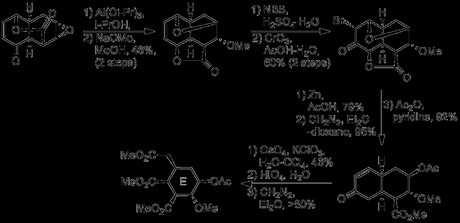
In fact, upon closer inspection, this tetracycle is not quite the perfect intermediate, as it contains an unwanted carbocycle, THF ring and lactone, as well as a couple of extra stereogenic centres, and Woodward now set about trimming it down to the required size. This process began with bromohydrin formation at the remaining olefin, with bromonium formation (on the convex face) followed by regioselective opening by water, possibly assisted by hydrogen bonding to the nearby lactone oxygen. Oxidation of the bromohydrin hydroxyl group gave the α-bromoketone, which underwent another impressive cascade sequence upon treatment with zinc in acetic acid, resulting in both the tetrahydrofuran and lactone rings being opened.[7] Esterification of the liberated acid with diazomethane and acetate protection of the freed hydroxyl then gave a bicyclic enone, with just the five E-ring stereocentres remaining. The rigid decalin system had now outlived its usefulness, having admirably performed its role in guiding stereoselective oxygenation around the molecule. Thus, the unwanted cyclohexenone was removed by first dihydroxylation with osmium tetroxide, followed by oxidative cleavage to the diacid, which was converted to the corresponding diester by treatment with diazomethane. From here, only 9 steps including a resolution were required to complete the natural product, and you can read about those here next Wednesday!
Etc
- The most recent review is a little dated now: Chem. Rev. 2005, 105, 4671.
- By my count that’s 13.5 litres of pyridine, 30 gallons of ether (~113 litres) and 8 gallons (~30 litres) of benzene. For one step. Not to mention the 70 litres pentane, 30 litres methanol and 30 litres acetone used in the work up. That sounds like an awful lot to be slinging around an academic lab... heck, in the lab where I work I believe the limit for total amount of flammable liquids we’re allowed (including solvent, washing up acetone and waste) is 50 litres!
- My institution doesn't have an electron subscription to Tetrahedron reaching that far back, so I went and lovingly photocopied all 60 or so pages out of the library one lunch break a few years ago. It was totally worth the effort. I typed up this whole extract by hand, and am sorry for any mistakes.
- I can’t resist linking to my other occasional blog, blog-syn, here (although it was Vinylogous’s idea, and See Arr Oh does most of the work). Incidentally, I have repeated Woodward’s procedure for this Diels-Alder (on 1/100th of the scale, i.e. 30 g) and also several steps from his synthesis of 6-methoxytryptamine, both from this paper, and even on a drastically different scale I found both to be extremely reproducible. I half-wrote a blog post on this a year back (tentatively titled “In Our Hands…”), but then had second thoughts about giving away too much detail here about what I do, especially if it might get published. It’s not looking that way at the moment, though, so maybe I’ll finish it off and put it up one day!
- It’s surprising how much common terminology comes from footnotes in Woodward papers. It’s how I first learnt of Linstead notation, and it turns out that the now ubiquitous terms convex and concave (as applied to bicyclic systems) were also first defined in this way: “The term convex face is used in the sequel for that side of cis decalin derivatives on which the cis bridgehead hydrogen atoms are located. The use of this designation, and the complementary concave face for the opposite side, obviates the ambiguities inherent in such phrases as “from the top side,” and “attack from the rear.””.
- Woodward’s explanation for this is interesting, and invokes the kind of orbital overlap responsible for the norbonenyl cation, supported by a citation to some earlier solvolysis studies he carried out with the father of non-classical cations (and hence arch-enemy of H. C. Brown), Saul Winstein.
- I’ve seen a number of mechanisms given for this step, and the precise order of events wasn't determined by Woodward, but whatever pathways were travelled, the overall yield was excellent.
References
J. Am. Chem. Soc. 1956, 78, 2023
J. Am. Chem. Soc. 1956, 78, 2657
Tetrahedron 1958, 2, 1
