Okay, this second post is a lot later and a fair bit shorter than I had hoped it would be, but it's been a crazy and not entirely pleasant month. Enjoy!
In the previous Woodward Wednesday post I showed you guys the first half of Woodward's epic total synthesis of the popular bioactive natural product reserpine. If you didn't catch that when it came out, go and check it out, because I'm just going to carry straight on where it left off. Here goes!
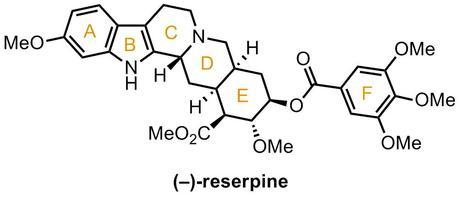
With the entire E-ring with its five contiguous stereocentres synthesised, albeit in racemic form, only a single stereocentre remained to be set, and this was to be done in the tetrahydrocarboline forming step. Thus, 6-methoxytryptamine was condensed with the E-ring aldehyde and the resulting iminium was then reduced with sodium borohydride to give a secondary amine that spontaneously cyclised onto the nearby ester to form the lactam. When this lactam was heated with a healthy excess of phosphorus oxychloride it underwent a Bischler-Napieralski-type cyclisation with the indole to form a dihydro-β-carboline that was immediately reduced with sodium borohydride before it could aromatise. Unfortunately, although the yield over these two steps was good, it was discovered that in the reduction that hydride had been added to the wrong face of the iminium ion, giving solely the unwanted diastereomer of the product.
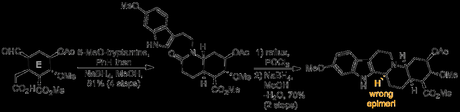
Epimerising this rather unreactive looking stereocentre, in the presence of all the other functionality in the molecule, was a daunting prospect, further complicated by the fact that the product obtained appeared to be the thermodynamic one. Although this result appeared disastrous at this stage in the campaign, Woodward reasoned that if the conformation of the D and E rings could be changed then the thermodynamic preference of the troublesome center might also change, making epimerisation possible. With this in mind, an extra ring was introduced into the system by hydrolysis of the acetate protecting group and methyl ester, followed by transannular lactone formation across the E-ring. Amazingly, this had the dramatic effect of forcing the C- and D-rings into a conformation that now allowed for acid-catalysed epimerisation of the final stereocentre.[1]
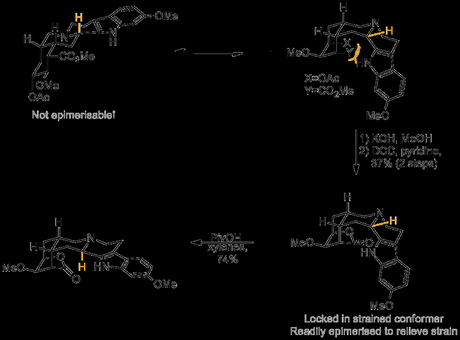
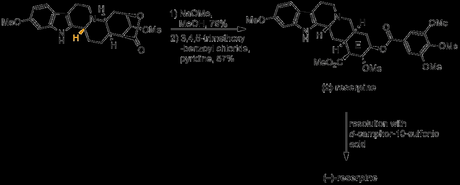
With this now set with the correct configuration the temporary lactone was now hydrolysed, and the trimethoxybenzoate ester found in the natural product was formed, producing racemic reserpine. Finally, resolution with D-camphorsulfonic acid gave a single enantiomer of the natural product!
Overall, I think this one of the finest total syntheses I've ever see; the use of simple reagents and substrate control to establish the required stereocentres around the E-ring is just breathtakingly clever,[2] the experimental is gorgeous (I've personally repeated several of the steps) and the discussion in the full paper is a joy to read. Go and check it out!
Etc
1. I was going to draw out a mechanism for the epimerisation, but I think that it's quite easy to see. Start off by protonating the indole (at C-3, of course), then the offending proton is acidic enough to be lost in tautomerisation of the indolenine.
2. I once set a problem session where I just redrew the first 10 or so steps of this synthesis starting from the Diels-Alder reaction, but with no stereochemical information at all – everything drawn flat. The idea was that people should use their knowledge and concave/convex bicycles to figure out how which side of the decalins each reaction occurred from. It was met with mixed reactions. You can check out here, if you're interested.
