As a synthetic chemist I'm always on the hunt for interesting molecules to disconnect/speculate about, and a couple of natural products published at the start of this month (Organic Letters ASAP; DOI: 10.1021/ol3028303) immediately caught my eye:
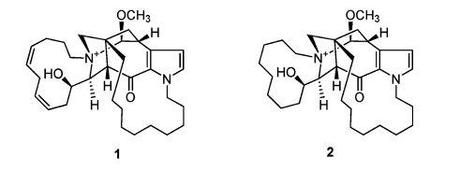
So far so good! Don't start looking for mistakes yet! The problems start when the authors go on to suggest what I'm going to politely call a most intriguing biosynthetic proposal:
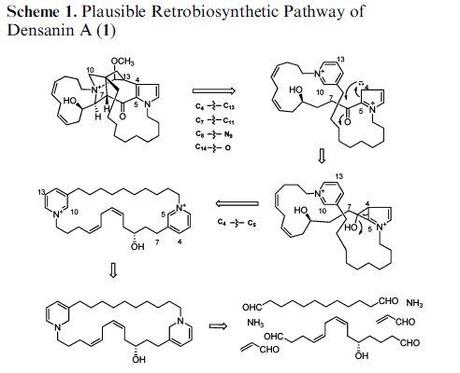
It's not too bad at the start, borrowing heavily from Baldwin and Whitehead's hypothesis for manzamine and related alkaloids. The problems come one the authors need to go from the pyridinium dimer to the natural product itself. How the C4-C5 bond is supposed to form on the middle line is beyond me, and the arrows in the cyclopropane opening seem to go in entirely the wrong direction, but all that stuff seems entirely sensible compared to the madness on the top line. I know that people often play a bit fast and loose with steps in proposed biosyntheses; it's easy to shrug and go "there's probably an enzyme that does that", but this just shows no understanding whatsoever of arrow pushing. I can't believe this got into an ACS journal, or am I missing something?
