In a recent group meeting the old Woodward aphorism came out again: "the only model system worth using is the enantiomer", which led to a scrabble afterwards to find when and where he'd actually said it. To my annoyance, I knew I'd undertaken the same search at the start of my PhD—and had all but given up until someone on Twitter helped me out. However, as I'd unfortunately lost the reference with the death of my old laptop, I again spent a considerable amount of time tracking it down again, for at least the second or third time in my life. Thus, as a favor to my future self—and in case anyone else is interested—I'm documenting the origins of the phrase here.
The quote itself most likely comes from a remark made by Woodward during a lecture he gave in London in 1968 on his progress towards the synthesis of vitamin B12. I'm probably not going to do a Woodward Wednesdays post on the B12 synthesis any time soon for reasons of time (as much as anything), but to give some context to the quote, a partial retrosynthesis is shown below. Woodward disconnected the molecule into eastern (B/C) and western domains (A/B), and set out to synthesise the western domain from the tricyclic indoline shown. Although B12 would be a daunting molecule to synthesis even diastereoselectively today, Woodward's aim was in fact to devise a route to the target in its natural, enantioenriched form—which in the 1960s meant either a dip in the chiral pool, or a resolution. Although the group was able to develop a route to either enantiomer of the slightly later intermediate XXXVII, starting from (+)- or (-)-camphor, for the final sequence they found that it was in fact more efficient to instead use a resolution of the earlier indoline, accomplished by derivatisation with (S)-α-phenylethyl isocyanate and separation of the resulting diastereomers.
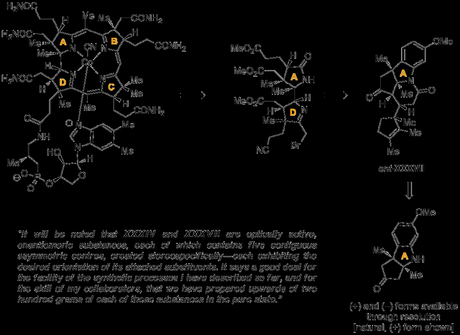
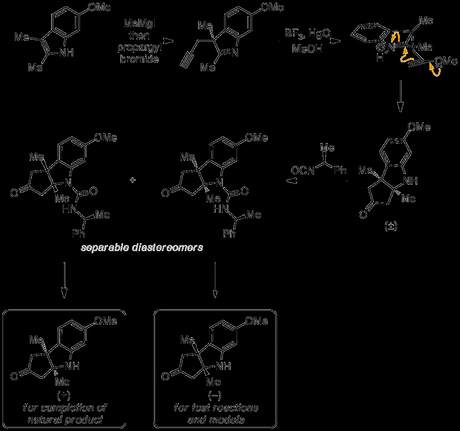
At first glance, resolution at this stage seems like an odd choice, because although this intermediate could be prepared very efficiently—even on a scale of several hundred grams—it was just 2 steps into a route of over 70 steps! Normally, when resolution is being used in a synthesis it's left as late as possible in the route to avoid using large quantities of a potentially expensive resolving agent, and because such separations can be fairly challenging on scale. However, as an old and wise professor once told me, there are generally two important considerations when performing such a separation: "resolution should be done 1. early; and most importantly 2. when actually possible"—and it was probably the second point that dictated proceedings here. Perhaps haunted by the difficult resolution at the end of his chlorophyll synthesis—a campaign that was in many ways a practice run for B12—and with an effective method using a a cheap reagent available, Woodward went for it!
However, as this method of separating the enantiomers allowed both to be recovered in essentially enantiopure form, the group now had an equal amount of both the building block for the natural series and its antipode. One criticism that can be made of resolutions is that half of the material is often wasted, but that's not what happened here; with another 68 steps left to work out the group had now the perfect model system, where any reactions performed were guaranteed to carry over to the real system! Woodward put it thusly:
Perhaps also this is the point at which I should emphasize explicitly the importance of the availability of the "unnatural" enantiomer (XXXVII). Much as had been our progress at this point, we were not unaware that we still had far to go, and that it might be either necessary or desirable—as indeed it turned out to be—to investigate a considerable number of alternatives for further advance. In these explorations we were able to utilize XXXVII, confident that whatever new route we might establish through its study would be applicable to its counterpart (XXXIV) of the natural series; our experience has been such that this is just about the only kind of model study which we regard as wholly reliable! And in fact, although the reactions I shall describe in the sequel will be presented for compounds in the natural series, almost all of them were first discovered using the enantiomeric substances. —R. B. Woodward in Pure Appl. Chem. 1968, 17, 519 (transcript of a lecture given at the 5th International Symposium on the Chemistry of Natural Products, London, UK, 8–13 July 1968; you can guess the title).[1]
Etc
1. As an aside, the earlier years of Pure and Applied Chemistry are a real treasure trove of chemical history. The journal has recently added a Nobel Laureates page, containing links to all the articles in the journal written by previous winners of the prize for chemistry (some 56 of 157 are represented). Although some of these are just fairly unremarkable, non-ground breaking research articles that didn't make it into JACS, there are also some real gems mixed in, mostly in the form of perspectives and lecture transcripts. Picking out all my favourites may well be the subject of a future post, but here are a few classics:
- H. C. Brown's retrospective on the non-classic ion debate that dominated physical organic chemistry for 30 years (and which he was rather on the wrong side of).
- Sir Alexander Todd speculating on the future of natural products synthesis in 1961
- Linus Pauling on the nature of metals. I agree with what Derek wrote this week; Pauling was a fantastic chemist, who unfortunately lost the plot a bit at the end.
- Vladimir Prelog on conformational analysis and medium rings.
- Charles Pederson—possibly the only Chemistry Nobel Laureate to not possess a PhD—on his discovery of the crown ethers
And there are many more from organic giants like E. J. Corey, R. B. Woodward, Sir Derek Barton and Sir Robert Robinson through to Noyori, Yonath, Ertl, Natta, Schrock, Debye, Seaborg and many others. And they're all available online for free!
