
The Ogasawara Archipelago (Bonin Islands,) encompasses several tens of small islands ~ 1000 km from mainland Japan. In 2011, UNESCO declared this archipelago a World Heritage Site. Some regard them as the “Galapagos of the Orient”, owing to their biological singularity, e.g., endemism rates of ~ 50 % of > 500 species of plants, or ~ 90 % of > 100 species of terrestrial snails. Photos show patches of native scrub (left) and introduced sheoak forest (right), close-ups of the two study species Ogasawarana discrepans (left) and O. optima (right), and empty shells with (top right, bottom) and without (top left) rat scars (Courtesy of Satoshi Chiba).
Another great post by Salvador Herrando-Pérez that challenges our views on invasive species (some would do well to heed his words when it comes to species like dingos). I mentioned in his last post that he had just recently submitted his PhD thesis, and now I’m proud to say that it has been examined with no recommended changes required. What a truly rare accolade. Congratulations, Salva.
–
A blunt instrument of ecological restoration is the elimination of introduced species. However, when introduced species become custodians of native wildlife, a dilemma emerges between re-establishing historical ecosystem conditions or instead, accepting foreign species for the benefits they might also bring.
Right after birth, we all enter a culture where what is ‘good’ or ‘bad’ has already been determined. Later on, if those values remain unchallenged, individuals assume them to be true and act accordingly (which is neither ‘good’ nor ‘bad’ necessarily… it is just so). Science is therefore the only recourse humans have to check such values by reducing the subjectivity of our judgements about why natural phenomena occur.
But scientists also work in a context of ‘pre-established truths’ (because, believe it or not, most of us are human too). The late Larry Slobodkin referred to our professional biases as ‘reifications’; i.e.,
“…reification consists of accepting a designation as if it has empirical meaning when, in fact, its existence has either never been tested or it has been found empty” (1).
Slobodkin underlined invasive species as an icon of reification. Indeed, people (with and without a scientific background) tend to demonise species that are not native and extremely abundant – experts even debate whether this is another sort of xenophobia (2). Thus, zebra mussels (Dreissena polymorpha), cane toads (Rhinella marinus) or caulerpa algae (Caulerpa taxifolia) are commonly referred to as ‘alien’, ‘invasive’ or ‘noxious’. Technically, we now call them ‘biological pollution’ (3). Such epithets are loaded with moral and pejorative connotations to qualify organisms that affect the range of facets of human well-being (aesthetics, economy, ethics, health).
In contrast to reification, several recent reviews have examined the positive ecological effects that introduced species can exert in the remote areas they manage to colonise (4, 5) (Table 1). Those effects can include how foreign organisms
- become habitat, shelter or food for native species,
- resume the functional roles of now-extirpated species, and/or
- constitute an ecosystem service.
–
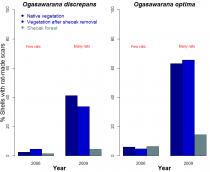
Figure 1. Mortality rates of two terrestrial snails (O. discrepans, O. optima) under the interaction of two introduced species (drooping sheoaks and black rats) on Ani-jima Island (Ogasawara Archipelago, Japan) (6). Snail mortality estimated as the percentage of empty shells showing rat-made scars (see photo) in years of low (2006) and high (2009) rat densities. Bar height indicates average mortality at several sites within three vegetation types: native, introduced sheoak forest, and secondary vegetation after removal of sheoaks. For both molluscs, mortality was < 10 % in 2006 across vegetation types. Contrastingly, the population boom of rats in 2009 resulted in 30 to 60 % higher snail mortality in vegetation with than without sheoaks. The layer of leaves and debris shed by sheoak trees seems to protect the snails from their rodent enemies.
As an example, Satoshi Chiba investigated the introduction of a tree and a mammal on the Island of Ani-jima (6). This island hosts 32 species of terrestrial snails, which are all endemic to the Ogasawara Archipelago and eaten by the introduced black rat (Rattus rattus). Despite being one of the best conserved islands of Ogasawara, a fraction of Ani-jima’s natural grasslands and scrubs have also been replaced by Australian drooping sheoaks (Allocasuarina verticillata). Over three years, Chiba showed that snail mortality rates by rats were up to 60 % higher in native vegetation than in sheoak forest (Fig. 1). Drooping sheaoks seem to deposit a thick layer of leaves and twigs on the ground (absent in the local vegetation) which protects the snails from rat attacks. The reality is that sheoaks and rats are not ‘good’ o ‘bad’ per se; rather, their joint introduction might benefit some species and harm others.
New species, new interactions
Invasive species are one of the main drivers of modern extinctions globally (7), resulting in billions of dollars of damage to agriculture, cattle pastoralism, hunting, fisheries and human health (8). Even when non-native species are imported with good intentions, chances are that once established, they can become invaders, as shown by the long history of ecological success and disaster caused by introduced predators to control agricultural pests (7, 8).
However, because of the novel interactions emerging between introduced and native species whenever an invasion takes over, eradication might not be necessarily the ideal solution (9). For instance, following the elimination of the red fire ant (Wasmannia auropunctata) from Santa Fe Island (Galapagos, Ecuador), many native ant species recovered (10). In contrast, the southwestern willow flycatcher (Empidonax traillii extimus) turned to rely on tamarisks (Tamarix spp.) for nesting after these Eurasian trees replaced large strips of the arid vegetation in western USA (11).
These and many other examples (see Table 1) show us that in spite of their negative environmental impacts on average, our tolerance of already introduced species might increase over time insofar as we gradually acknowledge the benefits which they can sometimes provide.
Table1. Examples of positive effects of introduced biota on native species.
Introduced species Contrasting effects on native species Where Refs.
Honey bees Apis mellifera Outcompete native pollinators, but increase fertility of endemic shrub New South Wales Australia (12)
American crayfish Procambarus clarkii Spread pathogenic fungi depressing autochthonous crayfish, but propel population growth of breeding and wintering birds Guadalquivir Spain (13)
European weevil Rhinocyllus conicus Successfully control European thistle, then attack native thistles Canada & USA (14)
American guava Psidium guajava Replace native vegetation, but attract savanna/rainforest birds that disperse local plant seeds Western Province Kenya (15)
American matico Piper aduncum Outcompete native plant pioneer colonizers, but preferred food by native (butterfly) caterpillars Madang Papua New Guinea (16)
Wild boar Sus scrofa Attract eagles, eagles decimate island foxes, endemic skunks (outcompeted by foxes) recover from near extinction Channel Islands California, USA (17)
Japannese white-eye Zosterops japonicus Contribute to extinction of native avifauna, but become main pollinator of indigenous shrub Hawaii USA (12)
Australian tubeworm Ficopomatus enigmaticus Build lagoon reefs that propel native crabs which in turn deplete local invertebrates Cordoba Argentina (18)
Eurasian salt cedar Tamarix sp. Replace riparian vegetation, but become main nesting tree for endangered birds Colorado USA (11)
Literature
- L. B. Slobodkin, Evol Ecol Res 3, 1 (Jan, 2001)
- D. Simberloff, Biol Invas 5, 179 (2003)
- M. Elliott, Mar Poll Bull 46, 275 (Mar, 2003)
- L. F. Rodriguez, Biol Invas 8, 927 (Jun, 2006)
- M. A. Schlaepfer, D. F. Sax, J. D. Olden, Conserv Biol 25, 428 (Jun, 2011)
- S. Chiba, Conserv Biol 24, 1141 (Aug, 2010)
- J. S. Cory, J. H. Myers, Trends Ecol Evol 15, 137 (Apr, 2000)
- M. B. Thomas, A. J. Willis, Trends Ecol Evol 13, 325 (Aug, 1998)
- E. S. Zavaleta, R. J. Hobbs, H. A. Mooney, Trends Ecol Evol 16, 454 (Aug, 2001)
- S. Abedrabbo, in Exotic Ants: Biology, Impact, and Control of Introduced Species, D. F. Williams, Ed. (Westview Press, Boulder, Colorado, USA, 1994), pp. 219-239
- M. K. Sogge, S. J. Sferra, E. H. Paxton, Restor Ecol 16, 146 (Mar, 2008)
- C. L. Gross, Biol Conserv 102, 89 (Nov, 2001)
- Z. Tablado, J. L. Tella, J. A. Sanchez-Zapata, F. Hiraldo, Conserv Biol 24, 1230 (Oct, 2010)
- S. M. Louda, D. Kendall, J. Connor, D. Simberloff, Science 277, 1088 (Aug, 1997)
- D. G. Berens, N. Farwig, G. Schaab, K. Boehning-Gaese, Biotropica 40, 104 (Jan, 2008)
- V. Novotny et al., Ecol Entomol 28, 704 (Dec, 2003)
- G. W. Roemer, C. J. Donlan, F. Courchamp, Proc Natl Acad Sci USA 99, 791 (Jan, 2002)
- E. Schwindt, A. Bortolus, O. O. Iribarne, Biol Invas 3, 137 (2001)

