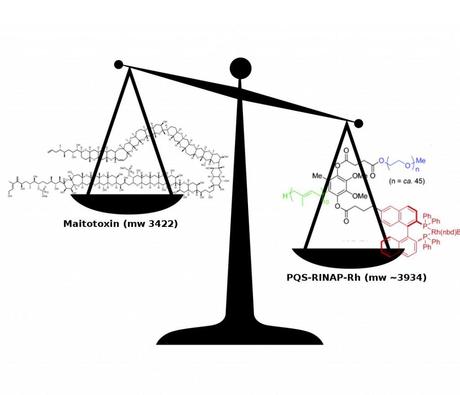
While browsing through the Advanced Synthesis and Catalysis Early View today, I noticed this paper on a new Rhodium catalyst for the asymmetric conjugate addition of boronic acids to enones in water. Pretty standard stuff... until you look at the catalyst structure. Now, I'm not particularly up on phosphine ligand design but I don't think that PEG and geranyl geranyl geranyl geranyl geranyl chains are a common thing to include. I mean, I've seen BINAP derivatives with some pretty big polyaromatic hydocarbon groups bolted on (e.g. anthracene and pyrene), but this is in a class of its own. In fact, I think that I can safely say that this is the largest catalyst for anything that I've ever seen. I guess the logic is that if you can't have the organic solvent in the flask you can just include it in the catalyst itself by larding it with hydrocarbon and polyether chains, and it does actually seem to work. A possible drawback of this approach is that the darn thing weighs more than maitotoxin and takes a total of twelve steps to make; all that just so we can have another way to add phenylboronic acid to cyclohexenone. I'm making a note of this for the next time someone calls my total synthesis project useless. Or am I being too harsh?
