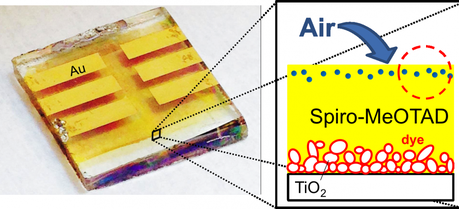
 Air exposure causes incorporation of gas molecules into the spiro-MeOTAD layer. (Credit: OIST)
Air exposure causes incorporation of gas molecules into the spiro-MeOTAD layer. (Credit: OIST)Researchers at the Okinawa Institute of Science and Technology (OIST) have made a surprising discovery about the degradation of dye-sensitized solar cells that could help pave the way to prolonging the lifetime of these cells.
Professor Yabing Qi and members of his unit have investigated the cause of dye-sensitized solar cell degradation. This discovery, published in The Journal of Physical Chemistry Letters (see footnote), can help move various forms of solar cell technology forward now that researchers know what is causing degradation and shortening the lifespan.
Solid state dye-sensitized solar cells have shown their potential in achieving high efficiency with a low cost of fabrication. Degradation of these cells, which shortens lifespan, is not well understood. To investigate the causes of degradation, Prof. Qi and colleagues focused on a material widely used in these solar cells, which is abbreviated spiro-MeOTAD. This material is used in the upper-most layer of the solar cell and comes into contact with the outside environment. Therefore, it is a likely candidate to be susceptible to degradation from many possible sources including air exposure, continuous light irradiation, elevated temperature and dust.
The most likely source of degradation was thought to be photo-oxidation, which is a chemical process caused by exposure to both air and light. Prof. Qi and colleagues tested whether this process was occurring. Surprisingly, they showed conclusively that there was no detectable photo-oxidation, or chemical degradation, of spiro-MeOTAD even after exposure for a few days. The researchers next looked at other possible dye-sensitized solar cell degradation mechanisms due to exposure to air alone. Spiro-MeOTAD is an amorphous substance, which is the property that makes it useful in these solar cells. However, this property could also cause a problem in that molecules from the air may easily diffuse, or freely pass into, the spiro-MeOTAD. These air molecules would then become impurities in the solar cells, leading to degradation. After a detailed analysis, the researchers determined this was precisely what was occurring; foreign air molecules were causing degradation of the spiro-MeOTAD layer, resulting in a drop in solar cell efficiency.
The research group that studies air exposure-induced gas molecule incorporation into Spiro-MeOTAD films. From left: Katsuya Luis Ono, Yabing Qi, Gueorgui Nikiforov and Yuichi Kato. (Credit: OIST)
The next step is to find a material to encapsulate and protect the spiro-MeOTAD layer from air exposure and prevent diffusion and the subsequent degradation from occurring. Prof. Qi says, “if we can find a method of low cost encapsulation, it is possible, for the first time, to achieve low cost, high efficiency and long lifespans in the same cell.” Since these solar cells are easy and cost-efficient to produce, adding this extra step can provide one more piece of the puzzle for an ideal solar cell.
Prof. Qi added, “this technology is compatible with the coating technology of the flexible transparent electrodes that we are also working on, meaning we can use this research for fabrication of large area, transparent, flexible solar cells.”
This work was performed in collaboration with Professor Antoine Kahn and his research group at Princeton University. DSSC, Advanced Technologies R&D, Merck Ltd., Japan kindly provided spiro-MeOTAD for this study.
Ono, L., Schulz, P., Endres, J., Nikiforov, G., Kato, Y., Kahn, A., & Qi, Y. (2014). Air-Exposure-Induced Gas-Molecule Incorporation into Spiro-MeOTAD Films The Journal of Physical Chemistry Letters, 1374-1379 DOI: 10.1021/jz500414m
