 Female green turtles (Chelonia mydas) spawning (top) and diving (bottom) on Raine Island (Great Barrier Reef, Queensland, Australia) — photos courtesy of Ian Bell. This species is ‘Endangered’ globally since 1982, mainly from egg harvesting (poaching conflict in Mexico for olive ridley Lepidochelys olivacea featured by National Geographic’s video here), despite the success of conservation projects (39). Green turtles inhabit tropical and subtropical seas in all oceans. Adults can grow > 150 kg and live for up to ~ 75 years. Right after birth, juveniles venture into the open sea to recruit ultimately in coastal areas until sexual maturity. They then make their first reproductive migration, often over 1000s of km (see footage of a real dive of a camera-equipped green turtle), to reach their native sandy beaches where pregnant females will ultimately lay their eggs. Each female can deposit more than one hundred eggs in her nest, and in several clutches in the same season because they can store the sperm from multiple mating events.
Female green turtles (Chelonia mydas) spawning (top) and diving (bottom) on Raine Island (Great Barrier Reef, Queensland, Australia) — photos courtesy of Ian Bell. This species is ‘Endangered’ globally since 1982, mainly from egg harvesting (poaching conflict in Mexico for olive ridley Lepidochelys olivacea featured by National Geographic’s video here), despite the success of conservation projects (39). Green turtles inhabit tropical and subtropical seas in all oceans. Adults can grow > 150 kg and live for up to ~ 75 years. Right after birth, juveniles venture into the open sea to recruit ultimately in coastal areas until sexual maturity. They then make their first reproductive migration, often over 1000s of km (see footage of a real dive of a camera-equipped green turtle), to reach their native sandy beaches where pregnant females will ultimately lay their eggs. Each female can deposit more than one hundred eggs in her nest, and in several clutches in the same season because they can store the sperm from multiple mating events.When sex is determined by the thermal environment, males or females might predominate under sustained climatic conditions. A study about marine turtles from the Great Barrier Reef illustrates how feminisation of a population can be partitioned geographically when different reproductive colonies are exposed to contrasting temperatures.
Fortunately, most people in Western societies already perceive that we live in a complex blend of sexual identities, far beyond the kind of genitals we are born with. Those identities start to establish themselves in the embryo before the sixth week of pregnancy. In the commonest scenario, for a human foetus XY with one maternal chromosome (X) and one paternal (Y) chromosome, the activation of the Srygen (unique to Y) will trigger the differentiation of testicles and, via hormonal pathways, the full set of male characteristics (1).
Absence of that gene in an XX embryo will normally lead to a woman. However, in just one of many exceptions to the rule, Sry-expression failure in XY individuals can result in sterile men or ambiguous genitals — along a full gradient of intermediate sexes and, potentially, gender identities. A 2015 Nature ‘News’ feature echoes two extraordinary cases: (i) a father of four children found to bear a womb during an hernia operation, and (ii) a pregnant mother found to host both XX and XY cells during a genetic test – with her clinical geneticist stating “… that’s the kind of science-fiction material for someone who just came in for an amniocentesis” (2). These real-life stories simply reflect that sex determination is a complex phenomenon.
Three ways of doing it
In nature, there are three main strategies of sex determination (3) — see scheme here:
- Hermaphroditism: when an individual has male andfemale organs through a life’s time. We see this, for example, in many flowers with ovaries and stamens (simultaneous hermaphrodites), or in many fish born one sex but dying the other (sequential hermaphrodites).
- Genetically driven: when genetic composition guides the path to body feminisation or masculinisation. Through the tree of life, one encounters an amazing panoply of variants. For example, in birds two identical chromosomes are carried only by males (symbolised by ZZ), so one of the female chromosomes (ZW) determines sex in our feathery cousins; males or females are born from unfertilised or fertilised eggs in some bees; separate sexes occur only in the phase of spores in mosses; two types of females specialise in either male or female offspring in some crustaceans and flies, let alone the multiple examples where sex is determined by multiple genes, parasites or the mitochondria (3).
- Environmentally driven: when the sex of the progeny depends on the external habitat. Thus, in the green spoonworm (see video) larvae settling on the seafloor develop as females and larvae landing on adult females ultimately become tiny males living inside their partner; and in many reptiles, the temperature outside the egg determines sex as embryonic development marches on.
Beach incubators
Reptiles are arguably the most complex vertebrate group in this respect (4). They can exhibit environmental or genetic sex determination, and also constitute excellent models to study sex allocation as a driver of evolution (5-7). For instance, some Mesozoic marine reptiles might have evolved gene determination to bypass the constraints of the stable thermal environment of the open ocean (8). Modern species can switch from genotypic to environmental sex determination in response to increased habitat temperatures (9); sex chromosomes and incubation temperatures can jointly shape the balance of sexes (10), and transitions back and forth among sex-determining strategies have been unveiled (7, 11).
In conservation biology, sex determination — particularly in marine turtles — has attracted a lot of attention since the 1990s due to climate change. Many might be familiar with the classic wildlife-documentary scene of a gigantic turtle crawling heavily ashore to bury her eggs under the sand (see wonderful nesting and hatching footage). Mum gets back to sea and, thereafter, the embryos’ growth is plugged to the warmth of the sun, such that if sand temperatures exceed, or not, a thermal threshold called a ‘pivotal temperature’ (see Table 1), then female or male offspring should predominate (12), respectively. So the fact that sex determination depends on nest temperature, which in turn depends on insolation and environmental temperature, has long begged the question of how climate warming can condition sex in marine turtles, and hence their demographic future? (13-15) — a question nicely exemplified by this short WWF documentary in Costa Rica.
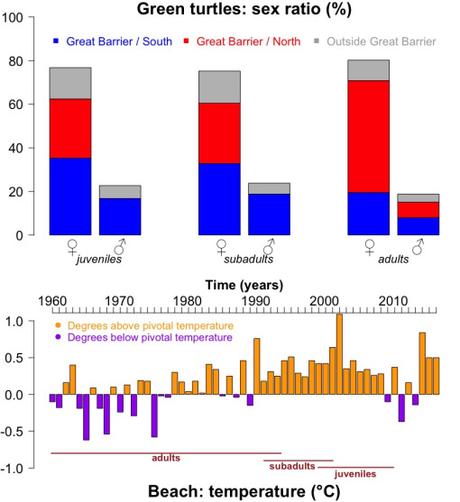
We can imagine a beach as a large incubator of turtle eggs, and a sequence of beaches along the coastline as a series of incubators provoking the birth of males or females according to the gradient of local temperatures. Jensen and collaborators (16) have studied this natural macrolaboratory in the Howick Group National Park (Queensland, Australia). These are a group of 19 Australian islands serving as foraging area for juveniles and adults of one of the largest populations anywhere of green turtles (Chelonia mydas), mostly breeding along the ~ 2500 kilometres of the Great Barrier Reef. Jensen sexed 411 individuals by examining their gonads via laparoscopy (see vet application in a marine turtle), and by measuring testosterone concentrations in blood.
They found that 7 of every 10 turtles turned out to be female. But most strikingly, they took skin samples to locate the birthplace for the study individuals — according to genetic proximity — in a DNA catalog of 25 nesting colonies. They found that virtually all non-adult congregating on the Howick Islands was a female if born along the northern Great Barrier (99% in juveniles and subadults; 87% in adults). In contrast, feminisation barely reached 70% among turtles native to the southern Great Barrier, irrespective of age. Climatic data gathered from the 1960s supported the notion that northern-born individuals had mostly hatched on beaches overheated above the pivotal temperature (29.3°C) as a result of climate warming (16). This thermal contrast, north and south of the Great Barrier Reef, is corroborated by coral-bleaching incidence exceeding 80% in the north, but below 33% in the south, as revealed by the latest comprehensive survey (17).
Table 1. Pivotal temperatures are thermal thresholds giving a 1:1 sex ratio in marine turtles — above/below which females/males predominate. They are tightly conserved around ~ 29 ºC, and within a range of 4 ºC, across the seven extant species.
marine turtle species country pivotal temperature (°C) IUCN Red List status
loggerhead (Caretta caretta) Australia 27-30 Vulnerable
Brazil 29-30
South Africa 29-30
leatherback (Dermochelys coriacea) Costa Rica 29-30 Vulnerable
French Guiana 29-30
hawksbill (Eretmochelys imbricata) Brazil 29-30 Critically Endangered
Guatemala 29-30
Puerto Rico 29-30 (18)
green (Chelonia mydas) Australia 29-30 Endangered
Costa Rica 28-30
Cyprus 29-30 (19)
Guinea-Bissau 29-30 (20)
Suriname 28-29
olive ridley (Lepidochelys olivacea) Costa Rica 28-31 Vulnerable
Kemp’s ridley (Lepidochelys kempii) Mexico 30-31 Critically Endangered
flatback (Natator depressus) Australia 29-30 (21) Data Deficient
Demographic conundrum
Feminisation has been detected globally in most of the largest populations of marine turtles (23). In principle, the fertility of a population is given by the number of offspring per female per unit of time (24), so theoretically female proliferation can boost reproductive performance in these reptiles (23, 25). Besides, inbreeding depression (tentatively caused by few males contributing to offspring) can be prevented by several mechanisms, e.g., (i) if individual males mate more often than females (26), (ii) if clutches comprise mating of single females with multiple males (27), or (iii) if different males contribute to different nests because they are laid at different times during the breeding season (28).
Reversal in female-biased ratios seems unlikely for two reasons: (i) evolutionarily: pivotal temperatures are mildly inherited and would have to increase by nearly 30 standard deviations (a lot!) to keep up with projections of climate warming of up to 4 °C by 2010 (29), and (ii) climatically: turtles return to their natal beaches to reproduce (30), so it is arguable whether their behavior can select for cooler beaches (31) or cooler microhabitats within a given area (32).
Female-biased sex ratios could be demographically dangerous if leading to complete feminisation (33, 34). However, many experts agree that sand temperature is the most deleterious thread of climate change to marine turtles while they are out at sea (35). Indeed, beach temperatures are reaching lethal temperatures, killing the embryos prior to hatching altogether (23, 36, 37), but see 38 — biased sex ratios then become unimportant. Plasticity of nesting phenology (nesting in cooler beachers or times of the reproductive season) does not seem to counteract those impacts (29, 31, 37), opening the Pandora’s box of human intervention, e.g., egg translocation or installation of beach shades; 35, 39).
There are many unknowns about the future viability of these magnificent animals — hopefully it is not too late once we know for certain.
Salvador Herrando-PérezDavid R. VieitesLiterature cited
-
- P. Berta et al. Genetic evidence equating SRY and the testis-determining factor. Nature 348: 448 (1990)
- C. Ainsworth. Sex redefined. Nature 518: 288-291 (2015)
- D. Bachtrog et al. Sex determination: Why so many ways of doing it? PLoS Biology 12: 1-13 (2014)
- J. J. Bull. Sex determination in reptiles. Quarterly Review of Biology 55: 3-21 (1980)
- E. Wapstra, D. A. Warner. Sex allocation and sex determination in squamate reptiles. Sexual Development 4: 110-118 (2010)
- S. D. Sarre, T. Ezaz, A. Georges. Transitions between sex-determining systems in reptiles and amphibians. Annual Review of Genomics and Human Genetics 12: 391-406 (2011)
- B. Capel. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nature Reviews Genetics 18: 675 (2017)
- C. L. Organ, D. E. Janes, A. Meade, M. Pagel. Genotypic sex determination enabled adaptive radiations of extinct marine reptiles. Nature 461: 389 (2009)
- C. E. Holleley et al. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523: 79-82 (2015)
- R. Shine, M. J. Elphick, S. Donnellan. Co-occurrence of multiple, supposedly incompatible modes of sex determination in a lizard population. Ecology Letters 5: 486-489 (2002)
- T. Gamble et al. Restriction site-associated DNA sequencing (RAD-seq) reveals an extraordinary number of transitions among gecko sex-determining systems. Molecular Biology and Evolution 32: 1296-1309 (2015)
- J. Davenport. Temperature and the life-history strategies of sea turtles. Journal of Thermal Biology 22: 479-488 (1997)
- L. A. Hawkes, A. C. Broderick, M. H. Godfrey, B. J. Godley. Climate change and marine turtles. Endangered Species Research 7: 137-154 (2009)
- F. J. Janzen. Climate change and temperature-dependent sex determination in reptiles. Proceedings of the National Academy of Sciences of the USA 91: 7487-7490 (1994)
- E. S. Poloczanska, C. J. Limpus, G. C. Hays. in Advances in Marine Biology. (Academic Press, 2009), vol. 56, chap. 2, pp. 151-211
- M. P. Jensen et al. Environmental warming and feminization of one of the largest sea turtle populations in the world. Current Biology 28: 154-159.e154 (2018)
- T. P. Hughes, J. T. Kerry, T. Simpson, Large-scale bleaching of corals on the Great Barrier Reef. Ecology 99: 501 (2018)
- N. Mrosovsky, S. J. Kamel, C. E. Díez, R. P. van Dam. Methods of estimating natural sex ratios of sea turtles from incubation temperatures and laboratory data. Endangered Species Research 8: 147-155 (2009)
- A. C. Broderick, B. J. Godley, S. Reece, J. R. Downie. Incubation periods and sex ratios of green turtles: highly female biased hatchling production in the eastern Mediterranean. Marine Ecology Progress Series 202: 273-281 (2000)
- A. R. Patrício et al. Balanced primary sex ratios and resilience to climate change in a major sea turtle population. Marine Ecology Progress Series 577: 189-203 (2017)
- S. Hewavisenthi, C. J. Parmenter. Hydric environment and sex determination in the flatback turtle (Natator depressus Garman) (Chelonia: Cheloniidae). Australian Journal of Zoology 48: 653-659 (2000)
- T. Wibbels. in The Biology of Sea Turtles, Volume II (2002), pp. 103-134
- G. C. Hays, A. D. Mazaris, G. Schofield, J.-O. Laloë. Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proceedings of the Royal Society B 284: 20162576 (2017)
- C. J. A. Bradshaw, C. R. McMahon. in Encyclopedia of Ecology, S. E. Jorgensen, B. Faith, eds. (Academic Press, Oxford, 2008), pp. 1535-1543
- P. Santidrián Tomillo, M. Genovart, F. V. Paladino, J. R. Spotila, D. Oro, Climate change overruns resilience conferred by temperature-dependent sex determination in sea turtles and threatens their survival. Global Change Biology 21: 2980-2988 (2015)
- G. C. Hays, A. D. Mazaris, G. Schofield. Different male vs. female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Frontiers in Marine Science 1: 1-9 (2014)
- J. A. Lasala, C. R. Hughes, J. Wyneken. Breeding sex ratio and population size of loggerhead turtles from Southwestern Florida. PLoS One 13: e0191615 (2018)
- N. Mrosovsky, S. R. Hopkins-Murphy, J. I. Richardson. Sex ratio of sea turtles: seasonal changes. Science 225: 739-741 (1984)
- J. M. Refsnider, F. J. Janzen. Temperature-dependent sex determination under rapid anthropogenic environmental change: Evolution at a turtle’s pace? Journal of Heredity 107: 61-70 (2016)
- P. T. Plotkin. in The Biology of Sea Turtles (CRC Press, 2009), pp. 225-241
- J. M. Refsnider, F. J. Janzen. Behavioural plasticity may compensate for climate change in a long-lived reptile with temperature-dependent sex determination. Biological Conservation 152: 90-95 (2012)
- G. C. Hays, A. C. Broderick, F. Glen, B. J. Godley. Climate change and sea turtles: a 150-year reconstruction of incubation temperatures at a major marine turtle rookery. Global Change Biology 9: 642-646 (2003)
- J. Patino-Martinez, A. Marco, L. Quiñones, L. Hawkes. A potential tool to mitigate the impacts of climate change to the caribbean leatherback sea turtle. Global Change Biology 18: 401-411 (2011)
- J.-O. Laloë, N. Esteban, J. Berkel, G. C. Hays. Sand temperatures for nesting sea turtles in the Caribbean: Implications for hatchling sex ratios in the face of climate change. Journal of Experimental Marine Biology and Ecology 474: 92-99 (2016)
- M. M. P. B. Fuentes, M. R. Fish, J. A. Maynard. Management strategies to mitigate the impacts of climate change on sea turtle’s terrestrial reproductive phase. Mitigation and Adaptation Strategies for Global Change 17: 51-63 (2012)
- L. A. Hawkes, A. C. Broderick, M. H. Godfrey, B. J. Godley. Investigating the potential impacts of climate change on a marine turtle population. Global Change Biology 13: 923-932 (2007)
- V. S. Saba, C. A. Stock, J. R. Spotila, F. V. Paladino, P. S. Tomillo. Projected response of an endangered marine turtle population to climate change. Nature Climate Change 2: 814-820 (2012)
- E. Abella Perez, A. Marco, S. Martins, L. A. Hawkes. Is this what a climate change-resilient population of marine turtles looks like? Biological Conservation 193: 124-132 (2016)
- A. D. Mazaris, G. Schofield, C. Gkazinou, V. Almpanidou, G. C. Hays. Global sea turtle conservation successes. Science Advances 3: 1-7 (2017)

