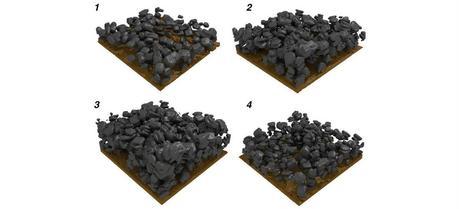
 Particles of a tin oxide electrode experiencing structural changes during charging (1-3) and discharging (3-4). (Graphic: Martin Ebner, Laboratory for Nanoelectronics, ETH Zurich)
Particles of a tin oxide electrode experiencing structural changes during charging (1-3) and discharging (3-4). (Graphic: Martin Ebner, Laboratory for Nanoelectronics, ETH Zurich)
One promising direction in lithium-ion battery research involves using material capable of alloying and de-alloying with lithium. This allows batteries to store more energy, however, their capacity decreases rapidly after a couple of charging and discharging cycles due to the electrode degradation. Researchers from the Swiss Federal Institute of Technology (ETH) in Zurich used X-rays to study the degradation process and find a way to prevent it.
Currently battery electrodes contain active materials known as intercalation compounds. These materials store charge in their chemical structure without undergoing substantial structural change. That makes these batteries comparatively long-lived and safe. However, intercalation materials have one drawback: their limited energy density, the amount of energy they can store per volume and mass.
In the search for higher energy density batteries, scientists have experimented for more than 20 years with materials capable of repetitively alloying and de-alloying with lithium. Laboratory-scale experiments have shown that batteries with such materials have energy densities multiple times that of intercalation materials; however, these alloying materials are not yet exploited in industry because their lifetime is limited. This is attributed to a massive—up to threefold—expansion of the electrode material during charging. During discharge, the materials contract again, but do not reach their original state. Electrode particles break apart, the electrode structure disintegrates, and the fragments loose contact to the rest of the cell.
To better understand this complex electrochemical and mechanical degradation of the electrode and to gain insight into how to develop better batteries, Martin Ebner, Ph.D. student at the Laboratory for Nanoelectronics in the Department of Information Technology and Electrical Engineering (D-ITET), and ETH-Professor Vanessa Wood, head of the Laboratory for Nanoelectronics at D-ITET, recognized the need to study a battery electrode during operation, using non-invasive methods. To do so, they turned to an imaging tool developed by ETH-Professor Marco Stampanoni. The spectrally pure and intense synchrotron X-ray radiation enables the fast acquisition of high-resolution X-ray images that can be computationally assembled into three-dimensional movies.
The researchers observed the inside of the battery as it charged and discharged over 15 hours. They gathered unique, three-dimensional movies that capture the electrode degradation mechanisms occurring in the battery and quantified the processes occurring within every particle for the thousands of particles in the electrode. The results of this study will be published in the journal Science; a preprint version is available online at Science Express (see footnote).
The data illustrate that tin oxide (SnO) particles expand during charging due to the influx of lithium ions causing an increase in particle volume. The scientists demonstrate that material lithiation happens as a core-shell process, progressing uniformly from the particle surface to the core. The material undergoing this reaction expands linearly with the stored charge. The X-ray images show that charging destroys the particle structure irreversibly with cracks forming within the particles. “This crack-formation is not random,” emphasizes Ebner. Cracks grow at locations where the crystal lattice contains preexisting defects. During discharge, the particle volume decreases; however, the material does not reach its original state again; the process is therefore not completely reversible.
The volume change of the individual particles drives expansion of the entire electrode from 50 micrometers to 120 micrometers. However, during discharge, the electrode contracts only to 80 micrometres. This permanent deformation of the electrode demonstrates that the polymer binder that holds the electrode together is not yet optimized for high volume expansion materials. This is critical for battery performance because deformation of the binder causes individual particles to become disconnected from the electrode and the battery loses capacity.
In addition to demonstrating that X-ray tomographic microscopy provides insight into morphological changes in the particles and electrodes, the researchers show that this technique can also be used to obtain quantitative and spatially resolved chemical information.
The researchers chose crystalline tin oxide as a model material because it undergoes a series of complex transformations also present in other materials, enabling deeper understanding into the behavior of a variety of battery materials. The insights provide the basis for developing new electrode materials and electrode structures that are tolerant to volume expansion. For Wood the results of this work indicate the benefit of using amorphous or nanostructured materials for electrodes instead of crystalline ones. “On the quest for new materials, one must also bear in mind that they are only of industrial interest if they can be produced in large quantities at a low cost. However, amorphous and nanostructured materials offer a sufficient playground for innovation.” emphasizes Wood.
Ebner M, Marone F, Stampanoni M, & Wood V (2013). Visualization and Quantification of Electrochemical and Mechanical Degradation in Li Ion Batteries. Science (New York, N.Y.) PMID: 24136360
