Another fine contribution from Salvador Herrando-Pérez (see previous posts here, here, here and here).
–
 Sometimes evolution fails to shape new species that are able to expand the habitat of their ancestors. This failure does not rein in speciation, but forces it to take place in a habitat that changes little over geological time. Such evolutionary outcomes are important to predict the distribution of groups of phylogenetically related species.
Sometimes evolution fails to shape new species that are able to expand the habitat of their ancestors. This failure does not rein in speciation, but forces it to take place in a habitat that changes little over geological time. Such evolutionary outcomes are important to predict the distribution of groups of phylogenetically related species.
Those who have ever written a novel, a biography, or even a court application, will know that a termite-eaten photo or an old hand-written letter can help rebuild moments of our lives with surgical precision. Likewise, museums of natural sciences store historical biodiversity data of great value for modern research and conservation1.
A notable example is the study of chameleons from Madagascar by Chris Raxworthy and colleagues2. By collating 621 records of 11 species of the tongue-throwing reptiles, these authors subsequently concentrated survey efforts on particular regions where they discovered the impressive figure of seven new species to science, which has continued to expand3 (see figure below). The trick was to characterise the habitat at historical and modern chameleon records on the basis of satellite data describing climate, hydrology, topography, soil and vegetation, then extrapolate over the entire island to predict what land features were most likely to harbour other populations and species. This application of species distribution models4 supports the idea that the phenotypic, morphological and ecological shifts brought about by speciation can take place at slower rates than changes in the habitats where species evolve – the so-called ‘niche conservatism’ (a young concept with already contrasting definitions, e.g.,5-7).
Many ecological and evolutionary predictions have been made from niche conservatism8, inter alia:
- species will shift their ranges tracking their climatic regime in response to climate change
- the spread of invasive species
- the exploitation of domesticated species might be limited by the climatic conditions of their native ranges
- moisture and temperature thresholds will restrict the distribution of species lineages that originate in arid, temperate and tropical environments
- competition within a community of species might explain the absence of entire guilds from their niches; and
- niche conservatism can be an important mechanism in both sympatric (as in the chameleon example) and allopatric speciation.
The allopatric scenario is most obvious at the Tehuantepec Isthmus, a 200-km strip of low-altitude forest and wetlands, carved into the Andes and stretching from the Atlantic to the Pacific coasts of Mexico. Sister species and subspecies of butterflies, birds and mammals have been found in the mountain ranges of the Sierra Madre north and south of the isthmus9. At much broader spatial scales, beech species occur within a tight thermal range, 18 ºC in summer, -1 to -4 ºC in winter, in temperate forests from north-eastern America (Fagus grandifolia) and northern Europe (F. sylvatica and F. orientalis). This suggests that different species have evolved in response to a similar climatic regime since the beech ancestor became fragmented > 10 million years ago10.
Niche conservatism in the above studies was based on a common test for ‘niche similarity’11, that is, testing whether one species’ distribution model predicted the record localities of a sister species better than expected by chance. Thus [in space], the occurrence points of the humming bird Atthis heloisa (north and west of Tehuantepec) predicted all or most occurrence points of A. ellioti (south and east of the isthmus) and vice versa9; and [in time] museum records of chameleons before 1988 predicted > 80 % of the records from most recent inventories in Madagascar2.
For better or worse, niche conservatism is reliant on a rather labile concept, that of niche12 [In fact, I have potentially overlapped ‘niche’ and ‘habitat’ in this article13]. Therefore, it is perhaps unsurprising that the heavily mathematical methods to examine niche conservatism differ considerably7,11, e.g., in how to relate and interpret phylogenetic and ecological similarity5,6,14,15.
Being a young concept with massive implications in explaining speciation and population change, we can expect that the study of niche conservatism will increase exponentially over the next decade, and hope that experts will do their best to produce joint publications where they bring consensus to definitions and mathematical treatment rather than emphasizing their individual points of view.
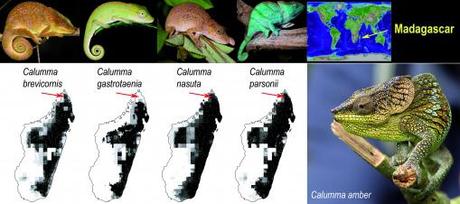
- The amber chameleon (Calumma amber) was discovered from cloud forests > 1000 m above sea level in the National Park of Montagne d’Ambre, Northern Madagascar (Ref. 3). Photo: Linda de Volder)
The type locality and first records of this species (red arrows) are located in an intersecting area of over-prediction of chamaleon habitat by means of ecological niche modeling based on satellite habitat data, museum records and recent surveys2. Shown niche distribution for four Calumma species, light to dark shading indicates improvement in niche features for those species (photos: Alyse de Vries and Thor Håkonsen). Madagascar hosts nearly half of the ~ 130 known species of chameleons, and phylogenetic data suggest that the world distribution of these reptiles in the African mainland, Europe and India radiated from Malagasy species through oceanic dispersal16. Nowadays, chameleons dwell in a variety of habitats from deserts to rain forests, where some taxa have kept their ancestral arboreal life and others have moved to live on the ground.
Literature cited1Graham, C. H., Ferrier, S., Huettman, F., Moritz, C. & Peterson, A. T. New developments in museum-based informatics and application in biodiversity analysis. Trends Ecol. Evol. 19, 497-503, doi:10.1016/j.tree.2004.07.006 (2004).
2Raxworthy, C. J. et al. Predicting distributions of known and unknown reptile species in Madagascar. Nature 426, 837-841, doi:10.1038/nature02205 (2003).
3Raxworthy, C. J. & Nussbaum, R. A. Six new species of occipital-lobed Calumma chameleons (Squamata: Chamaeleonidae) from montane regions of Madagascar, with a new description and revision of Calumma brevicorne. Copeia 4, 711-734, doi:10.1643/0045-8511(2006)6[711:SNSOOC]2.0.CO;2 (2006).
4Pearson, R. G. Species’ distribution modeling for conservation educators and practioners. Lessons in conservation 3, 54-89 (2010). <Available free at http://ncep.amnh.org/linc>.
5Losos, J. B. Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol. Lett. 11, 995-1003, doi:10.1111/j.1461-0248.2008.01229.x (2008).
6Pearman, P. B., Guisan, A., Broennimann, O. & Randin, C. F. Niche dynamics in space and time. Trends Ecol. Evol. 23, 149-158, doi:DOI: 10.1016/j.tree.2007.11.005 (2008).
7Wiens, J. J. et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecol. Lett. 13, 1310-1324, doi:10.1111/j.1461-0248.2010.01515.x (2010).
8Wiens, J. J. & Graham, C. H. Niche conservatism: Integrating evolution, ecology, and conservation biology. Annual Review of Ecology, Evolution, and Systematics 36, 519-539, doi:10.1146/annurev.ecolsys.36.102803.095431 (2005).
9Peterson, A. T., Soberón, J. & Sánchez-Cordero, V. Conservatism of ecological niches in evolutionary time. Science 285, 1265-1267, doi:10.1126/science.285.5431.1265 (1999).
10Huntley, B., Bartlein, P. J. & Prentice, I. C. Climatic control of the distribution and abundance of beech (Fagus L.) in Europe and North America. J. Biogeogr. 16, 551-560, doi:10.2307/2845210 (1989).
11Warren, D. L., Glor, R. E. & Turelli, M. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution 62, 2868-2883, doi:10.1111/j.1558-5646.2008.00482.x (2008).
12Soberón, J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol. Lett. 10, 1115-1123, doi:10.1111/j.1461-0248.2007.01107.x (2007).
13Whittaker, R. H., Levin, S. A. & Root, R. B. Niche, habitat, and ecotope. The American Naturalist 107, 321-338, doi:10.1086/282837 (1973).
14Losos, J. B. Rejoinder to Wiens (2008): Phylogenetic niche conservatism, its occurrence and importance. Ecol. Lett. 11, 1005-1007, doi:10.1111/j.1461-0248.2008.01232.x (2008).
15Wiens, J. J. Commentary on Losos (2008): Niche conservatism deja vu. Ecol. Lett. 11, 1004-1005, doi:10.1111/j.1461-0248.2008.01238.x (2008).
16Raxworthy, C. J., Forstner, M. R. J. & Nussbaum, R. A. Chameleon radiation by oceanic dispersal. Nature 415, 784-787, doi:10.1038/415784a (2002).


