Today's guest post is from Siddharth Yadav, an enthusiastic young chemist from somewhere in India. Enjoy!
I found B.R.S.M. when I was searching the web for the synthesis of cubane by Philip Eaton and was much delighted by the way the material was presented and interpreted, although a quick glance through B.R.S.M. showed me that this blog is not actually centred on compounds like cubane but rather on natural compounds (with their asymmetric carbons and stuff). So, I decided to write up a post on a compound that is much strained like the unnatural compounds but is indeed a naturally occurring chemical – pentacycloanammoxic acid.
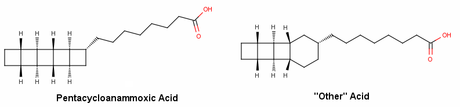
It all started when a guy named Damste discovered some unique lipids in some rare bacteria known as ‘Anammox’ (derived from Anaerobic Ammonia Oxidation) bacteria. These tiny guys oxidize ammonia and nitrite ions to liberate nitrogen gas and water, but during this conversion they produce hydroxylamine and hydrazine; two very damaging and membrane permeable intermediates! So as an SOS, these guys have a lipid bi-layer made of pentacycloanammoxic acid, which is denser than average membranes (dense enough to keep hydroxylamine and hydrazine at bay; hence avoiding their diffusion into the cytoplasm and preventing cellular damage).
Now to the really interesting part – structural determination of this ‘unique’ lipid gave a rather odd looking architecture! In fact they found two such lipids with slightly different structures. Much to the delight of the synthetic community; E. J. Corey and Vincent Mascitti jumped on the challenge for a total synthesis for pentacycloanammoxic acid. Any guesses why Corey and Mascitti didn’t choose the other acid?
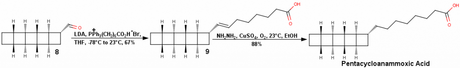
The Corey-Mascitti synthesis of racemic pentacycloanammoxic acid begins with CycloOctaTetraene (COT) which is brominated to give a brominated isomer of cyclooctatetraene (J. Am. Chem. Soc., 2004, 126, 15664, if you're interested –BRSM). The concentration of this brominated isomer will increase with time as the reaction is not readily reversible (no yield has been mentioned for this step by the authors, or it must have eluded me!). This brominated isomer then undergoes a hetro-Diels-Alder reaction with DBAD, which stands for DiBenzylAzoDicarboxylate and not for Di-tert-ButylAzoDicarboxylate and definitely not DiBenzylAcetyleneDicarboxylate! The alkene in Diels-Alder adduct 1 is then removed to give us saturated tricycle 2. This is then treated with Zinc in acetic acid to give compound 3, which undergoes a [2+2] photocycloaddition with 2-cyclopentenone to give 4 in adequate yields.
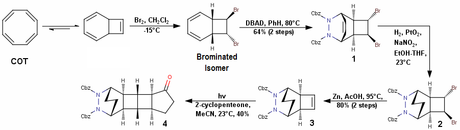
The next step actually tells us why the Diels-Alder was done in the first place; the Cbz group is removed by hydrogenation and the hydrazine thus obtained is converted to the azo compound 5 by the bubbling of oxygen through the solution. Does everyone know what happens next? You’re right – azo photolysis and transannular bond formation give us the complete pentacyclic framework of compound 6! However, the authors first decided to convert the carbonyl group to dimethoxyacetal (probably to protect it from the photolysis). This combination of hetero-Diels-Alder/oxidation/photolysis is exploited by many authors time and time again to form strained C-C bonds; another really clever use of this reaction can be seen in Thomas J. Katz’s synthesis of triprismane (see J. Am. Chem. Soc., 1973, 95, 2738 —BRSM).
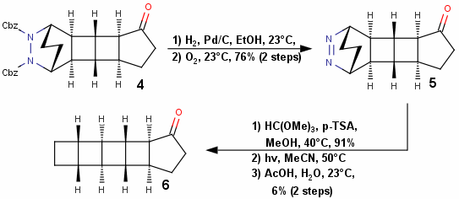
All that was now needed is a ring contraction and addition of the fatty acid tail, which surprisingly takes 8 more steps! That’s why I think the synthesis drags along at the end! The sequence used to get the desired ring contraction is Claisen condensation, diazo transfer and Wolff rearrangement.
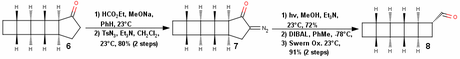
This compound 8 was then subjected to Wittig olefination with a pre-made phosphonium salt of the long chain carboxylic acid. The resulting alkene was then reduced using hydrazine, copper sulfate and with air as an oxidant i.e., diimide reduction to give pentacycloanammoxic acid in 0.25% overall yield. However, this synthetic strategy—needless to say—is racemic!
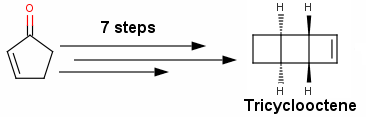
A second, enantioselective, synthesis was reported by the same authors some two years later (J. Am. Chem. Soc., 2006, 128, 3118 —BRSM). In contrast, this newer synthesis has an awkward initial sequence! Corey and Mascitti got to a certain compound called tricyclooctene in almost 7 steps—7 very painful and complicated steps—I feel that too much was done to get such a simple product! Maybe I am being too optimistic here but I guess most of you would agree that there should be a way to obtain tricyclooctene in less than 7 steps.
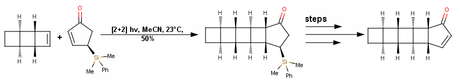
The key step in this second synthesis was a stereoselective [2+2] photocycloaddition! The principle is simple; the bulky group dimethylphenylsilyl group attached on the top face of enantioenriched cyclopentenone would cause the tricyclooctene to approach from the bottom face during the reaction. The product obtained from the photocyloaddition was then oxidized to the enone and stripped of the dimethylphenylsilyl group using TBAF to give the simple α,β-unsaturated ketone in 53% yield over three steps.
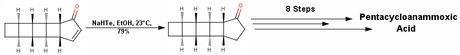
The next step was reduction of the alkene, but to everyone’s surprise the authors selected the “all exotic” method of hydrogenation; not using palladium-on-carbon or diazene reduction, but mono sodium telluride (NaHTe)! The compound hence obtained is actually the compound 6 of the first racemic synthesis, but this time in enantioenriched form. As you might have guessed, the end game is very similar (nearly same) to the first synthesis and takes about 8 steps to give pentacycloannamoxic acid in 1.9% (slightly better) overall yield.
The results of these two syntheses were: determination of structure and absolute stereochemistry, a route for the synthesis of this rare compound and most importantly the proud feeling that the chemical community gets when they know that they are a little closer to what Mother Nature can do.
For those of you interested in pentacycloanammoxic acid; here is some more info on such molecules. Compounds containing such linearly fused cyclobutanes are called ladderanes (due to obvious reasons!) and their syntheses have been achieved by three broad methods:- photochemical polycyclizations, thermal oligomerization and synthetic ingenuity (adding one ring at a time; as in the case of Corey and Mascitti’s synthesis). These syntheses as I see them; can be improved to a large degree with enough time and skilled minds, although there isn’t much rush to do so (if there is any at all!). But, as they say, “change is inevitable”, and a better synthesis of pentacycloanammoxic acid may come from someone reading this post, or even from the person who wrote it!
