 Guest post by Carina Baskett written in response to Angela Moles and Jeff Ollerton's post on Dynamic Ecology: Is the notion that species interactions are stronger and more specialized in the tropics a zombie idea?
Guest post by Carina Baskett written in response to Angela Moles and Jeff Ollerton's post on Dynamic Ecology: Is the notion that species interactions are stronger and more specialized in the tropics a zombie idea?Carina Baskett is a PhD candidate at Michigan State University in the Department of Plant Biology and the Ecology, Evolutionary Biology, and Behavior Program. She posts photos from her fieldwork and occasional articles about tropical natural history, among other things, at Wandering Nature.
 If you’ve traveled to the tropics, you know the drill. Get your shots for typhoid and yellow fever, and your meds for malaria (try to avoid the one with psychotic side effects). Don’t drink the water, and avoid the lettuce.
If you’ve traveled to the tropics, you know the drill. Get your shots for typhoid and yellow fever, and your meds for malaria (try to avoid the one with psychotic side effects). Don’t drink the water, and avoid the lettuce.This over-abundance of diseases and parasites in the tropics is not just because sanitation is lacking in developing countries. Both diversity and severity of human parasites are higher in the tropics (Cashdan 2001; Guernier, Hochberg et al. 2004).
Could the same be true for plant enemies? What about other biotic interactions, like predator-prey relationships, and plants and pollinators? And why?
The first person to suggest that biotic interactions are somehow different in the tropical and temperate regions was Alfred Russell Wallace. Not only did he independently conceive of natural selection around the same time as Darwin (during a malarial fever in Malaysia, speaking of tropical diseases!), he was also a great tropical naturalist.
In the book Tropical Nature in 1878, he said, “Equatorial lands must always have remained thronged with life; and have been unintermittingly subject to those complex influences of organism upon organism, which seem the main agents in developing the greatest variety of forms and filling up every vacant place in nature.”
The “biotic interactions hypothesis” to explain high tropical diversity* is a descendent of Wallace’s beautifully stated explanation, with contributions from Dobzhansky (1950), Fischer (1960), and Schemske (2009). At its core, skipping over tangents about coexistence and specialization, today’s conception has three testable parts:
- The relative contribution of biotic interactions to variation in relative fitness of organisms is greater at lower latitudes.
- Biotic selective agents drive faster divergence of allopatric populations than abiotic selective agents due to coevolution.
- Therefore, isolated populations speciate faster when the main selective agents are biotic.
I’ll focus the rest of this on part A, which was recently labeled a “zombie:” false, dead, disproven. I’ll try and convince you that it’s nowhere near dead yet. I’m NOT trying to convince you that the hypothesis is true, because I think the answer is very much up in the air, but rather that it’s plausible and that we need more data.
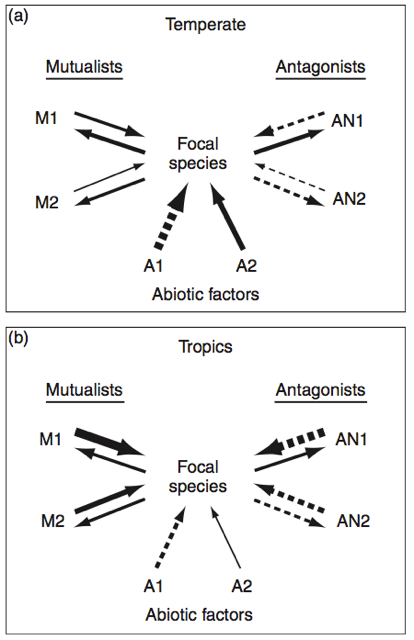 First, the dissection. Part A is represented graphically in Figure 1 (Schemske 2009). Each arrow is the proportion of variation in fitness for the focal species caused by different selective agents: mutualists, antagonists, and the abiotic environment. A wider arrow is a greater proportion. A solid line shows a positive effect on fitness, while a dashed line is negative. (Note that the arrows go both ways for the biotic agents. They coevolve, while abiotic agents do not, which gets into parts B and C of the hypothesis.)
First, the dissection. Part A is represented graphically in Figure 1 (Schemske 2009). Each arrow is the proportion of variation in fitness for the focal species caused by different selective agents: mutualists, antagonists, and the abiotic environment. A wider arrow is a greater proportion. A solid line shows a positive effect on fitness, while a dashed line is negative. (Note that the arrows go both ways for the biotic agents. They coevolve, while abiotic agents do not, which gets into parts B and C of the hypothesis.)Here’s what this abstract figure would look like on the ground. This past winter here in Michigan was, to put it lightly, a doozy. Two years ago, it was so mild that I was told that my first Michigan winter didn’t even count. It’s not hard to imagine that even after lineages have evolved to tolerate freezing (which is a big deal—it kills cells!), there can still be a lot of variation in fitness depending on the weather.
Think of a plant that lives for a few years, flowering in the summer, producing fruit in the fall, and dying back over winter. Let’s assume that there’s a resource tradeoff between manufacturing antifreeze and producing fruits.** This past winter, plants that invested a lot in antifreeze would have had high relative fitness in the population, because they alone survived. Two years ago, that same strategy would have had low relative fitness, because plants that produced less antifreeze would have survived the winter too, but had higher fruit production. In this hypothetical situation, there is some variation in fitness due to herbivores and pollinators etc., but most of it is due to the wildly variable weather.
In contrast, imagine a similar plant in a tropical habitat, growing in full sun. It experiences temperature stress too. The sunshine is actually more intense in the tropics because it’s hitting the Earth straight on instead of at an angle, so its energy is concentrated in a smaller area. It’s HOT! But it’s hot almost every single day of the year, so all the plants in the population invest in tolerating the heat to the same degree. Temperature isn’t contributing much variation in fitness in this population.
Life here is more like the Hunger Games. Who can grow the fastest? Who can avoid death by enemy herbivores and diseases? Who can form the strongest alliances with pollinators and fruit dispersers? Competition, antagonism, and mutualism is what determines fitness here, not the weather.***
Now that I’ve painted a picture of what Figure 1 could hypothetically look like in the real world, how can we figure out whether or not it’s true?
Ideally, you would pick a focal species and do an observational or experimental path analysis (e.g., Schemske and Horvitz 1988) to determine the relative contributions of various selective agents at different latitudes. Make sure you have an army of assistants, because you’ll need massive sample sizes. Did I mention you’ll have to do it for many years? You won’t want to miss important events like unusually bad winters or pest outbreaks. By the way, even though this kind of study would probably be impossible in animals, it’s also nigh-impossible to find an abundant plant species whose native range encompasses tropical and temperate latitudes, avoiding really dry places and high altitude. (If you know of one, please let me know!!)
Needless to say, filling in Figure 1 with real data has not yet been done, and given our funding climate, it probably never will. But, there are other ways to approximately test the hypothesis (Schemske, Mittelbach et al. 2009).
What we CAN ask is, what is the “intensity” of the interaction today? What do today’s traits tell us about selection in the past? And what is the frequency of an interaction in the community?
To illustrate each question in terms of pollination, we can ask how much do tropical plants rely on self-pollination vs. outcrossing; do tropical plants invest more in pollinator attraction and reward; and, do tropical plant communities show a higher frequency of animal vs. wind pollination? Fill in the blanks with your favorite interaction.
To build a relatively complete approximation of Figure 1, we should be asking these questions in many systems, across many types of interactions, and at many spatial and phylogenetic scales. For example, asking these questions within widely-ranging species (Salazar and Marquis 2012) is quite different from asking them at the community level, with disparate habitats, community membership, and growth forms (Moles, Wallis et al. 2011). Both approaches are needed, because each has huge advantages and severe limitations.
The most comprehensive review of the available data is a 2009 Annual Reviews paper by Schemske et al. (see their Table 1). They noted that the data was insufficient for meta-analysis. Nevertheless, they found that most interactions show greater “importance” at lower latitudes; that is, the interaction is more intense currently, the traits show that it was more intense in the past, or it is more frequent. None of the interactions shows greater importance at higher latitudes.
For example, in the tropics, predation rates on birds’ nests are higher. Ant predation rates on insect bait are higher. Parasite pressure is higher. Palatability of marine worms, salt marsh plants, leaves, and butterfly larvae is lower. The frequency of animal pollination, animal seed dispersal, ant-plant mutualisms, endophytes, and cleaning symbioses is higher. The review also finds that herbivory rates are higher and plants are better defended at lower latitudes, but a recent meta-analysis on herbivory came to different conclusions (Moles, Bonser et al. 2011).
Although the results are necessarily qualitative and we can’t put a p-value on this statement yet, this review shows that looking across many types of interactions, many ways of quantifying their importance, and over many spatial and phylogenetic scales, the weight of the available evidence supports Figure 1.
But I would be the last person in the world to tell you that we’re done testing part A of the biotic interactions hypothesis. Much of the available data was not generated to explicitly address it, so there’s always something missing. For example, herbivory rates could be the same at different latitudes, but tropical plants may be better defended, indicating that greater herbivore pressure has selected for stronger defense. You need both pieces of the puzzle, preferably measured at the same time on close relatives, to be able to say whether herbivore pressure is greater in the tropics.
I’ve spent the last three years thinking about how we can fill in the missing gaps. There are so many ways to test these questions, so many interactions and species to choose from. Each approach is limited in some key way; otherwise, the end-all, be-all experiment would have already been done! One could easily spend a lifetime chipping away at this question from different angles, without even addressing the bigger picture of whether this has anything to do with the latitudinal diversity gradient. (I’m working on that too though!)
Therefore, I was dismayed when I woke up on Tuesday to a post on a widely-read ecology blog that claimed that the hypothesis that biotic interactions are stronger in the tropics is a “zombie idea.” Meaning that it’s dead, it’s been disproven, we can all go home now, and anyone who studies it is just wasting their time.
Whoa. Not enough data for a meta-analysis, but we’re done with this question? A recent review concluded that there is support for the hypothesis, but now it’s been totally debunked? Did I miss something here?
In fact, I haven’t missed anything. As with any scientific controversy worth its salt, there are contradictory reviews, there are people who are highly skeptical, there is massive confusion about what the hypothesis is and how to properly test it. That’s all fine and good. It’s exciting, even.
What is not fine and good, in my book, is to proclaim from the rooftops that we’re done with a question that we’ve barely begun to address. To claim that a handful of publications on latitudinal gradients in herbivory are the end-all, be-all, period end of story of decades of research. To claim that any one of us has the final authority on how to define, test, and interpret an area of science.
A debate about an idea can be constructive and fun. But both my scientific and journalistic selves cry fowl when a story is presented hyperbolically from one point of view. I believe that it’s irresponsible and polarizing to instigate a debate by claiming that the problem is solved and there is no debate.
I’m glad that people are talking about the topic, though I wish it had been inspired by less inflammatory language. I hope the conversation inspires us to clarify what our questions are and how we can test them. I hope also that you agree that we don’t need more catchy metaphors (zombies, old clothes, lemmings, sheep). We need more data and more conversation. Period. But not the end of the story.
*There are so many species in the tropics! There are over 22,000 tree species in the Amazon, compared to 620 in temperate North America (Currie and Paquin 1987; Fine and Ree 2006). This pattern of higher species diversity in the tropics is remarkably consistent across different types of organisms and through time and space. How can the same underlying processes of ecology and evolution produce such different outcomes? We don’t really know! Sure, there are ideas. In fact, Palmer (1994) lists 120 hypotheses to explain it! But given the massive scale of space and time, it’s really hard to test these hypotheses, so a definitive answer remains elusive. See Mittelbach, Schemske et al. (2007) for a great review.
**For any of this to matter for evolution, we are also assuming that allocation strategies are not very plastic; that they are heritable; and that there is genetic variation for these strategies in the population.
***Why am I focusing so much on temperature? Lots of studies show that climatic variables, especially temperature, are tightly correlated with global diversity patterns (e.g., Currie, Mittelbach et al. 2004). In my opinion, for a few reasons, the biotic interactions hypothesis is the only latitudinal diversity gradient hypothesis that provides a plausible mechanism by which temperature can affect diversity. But actually, the hypothesis is generalizable to any gradient in abiotic stress. Although it was proposed to address the LDG, it applies to other gradients in abiotic stressors that covary with species diversity: altitude, ocean depth, precipitation, etc (Schemske, Mittelbach et al. 2009). This is a practical strength because components of the biotic interactions hypothesis can be tested in other systems, which may be more tractable than latitude. More importantly, it is a theoretical strength because confirming this hypothesis could revolutionize our approach to studying the origins of diversity in many systems.
References:
Cashdan, E. (2001). "Ethnic diversity and its environmental determinants: Effects of climate, pathogens, and habitat diversity." American Anthropologist 103(4): 968-991. Currie, D. J., G. G. Mittelbach, et al. (2004). "Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness." Ecology Letters 7(12): 1121-1134. Currie, D. J. and V. Paquin (1987). "Large-scale biogeographical patterns of species richness of trees." Nature 329(6137): 326-327. Dobzhansky, T. (1950). "Evolution in the tropics." American Scientist 38: 209-221. Farrell, B. D., D. E. Dussourd, et al. (1991). "Escalation of plant defense: Do latex and resin canals spur plant diversification?" American Naturalist 138(4): 881-900. Fearnside, P. M. (2005). "Deforestation in Brazilian Amazonia: History, rates, and consequences." Conservation Biology 19(3): 680-688. Fine, P. V. A. and R. H. Ree (2006). "Evidence for a time-integrated species-area effect on the latitudinal gradient in tree diversity." American Naturalist 168(6): 796-804. Fischer, A. G. (1960). "Latitudinal variation in organic diversity." Evolution 14: 64-81. Guernier, V., M. E. Hochberg, et al. (2004). "Ecology drives the worldwide distribution of human diseases." Plos Biology 2(6): 740-746. Jablonski, D., C. L. Belanger, et al. (2013). "Out of the tropics, but how? Fossils, bridge species, and thermal ranges in the dynamics of the marine latitudinal diversity gradient." Proceedings of the National Academy of Sciences of the United States of America 110(26): 10487-10494. Mittelbach, G. G., D. W. Schemske, et al. (2007). "Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography." Ecology Letters 10(4): 315-331. Moles, A. T., S. P. Bonser, et al. (2011). "Assessing the evidence for latitudinal gradients in plant defence and herbivory." Functional Ecology 25(2): 380-388. Moles, A. T., I. R. Wallis, et al. (2011). "Putting plant resistance traits on the map: a test of the idea that plants are better defended at lower latitudes." New Phytologist 191(3): 777-788. Palmer, M. W. (1994). "Variation in species richness: towards a unification of hypotheses." Folia Geobotanica & Phytotaxonomica 29(4): 511-530. Paterson, S., T. Vogwill, et al. (2010). "Antagonistic coevolution accelerates molecular evolution." Nature 464(7286): 275-U154. Salazar, D. and R. J. Marquis (2012). "Herbivore pressure increases toward the equator." Proceedings of the National Academy of Sciences of the United States of America 109(31): 12616-12620. Schemske, D. W. (2009). Biotic interactions and speciation in the tropics. Speciation and Patterns of Diversity. R. K. Butlin, J. R. Bridle and D. Schluter. Cambridge, United Kingdom, Cambridge University Press: 219-239. Schemske, D. W. and C. C. Horvitz (1988). "Plant-animal interactions and fruit production in a neotropical herb: a path analysis." Ecology 69(4): 1128-1137. Schemske, D., Mittelbach, G., Cornell, H., Sobel, J., & Roy, K. (2009). Is There a Latitudinal Gradient in the Importance of Biotic Interactions? Annual Review of Ecology, Evolution, and Systematics, 40 (1): 245-269 DOI: 10.1146/annurev.ecolsys.39.110707.173430.
