Thanks to Brandon and Martyn for pointing out these publications. Be warned, this post turned out seriously long and wordy!

Almost since the dawn of microwave chemistry, which began in the 1980s with people simply putting Erlenmeyer flasks full of reactants in domestic microwaves, chemists have reported all kinds of improvements from heating in this fashion. To name a few of the more common ones, I've heard people claim higher yields, shorter reaction times, cleaner reactions, different selectivities, milder conditions and better overall energy efficiency. Microwave chemistry can be a good thing, and many of these effects are real, widely observed phenomena; the problem is that chemists disagree on their origins. However, comparison between microwave and conventionally heated reactions is fraught with difficulties. One obvious factor is that microwave reactions, at least in the organic chemistry labs that I’ve worked in, are inevitably conducted in sealed tubes, which makes direct comparison to the ‘open’ systems that are typically used in conventional reaction set-ups. Heck, even in open systems, superheating of solvents past their boiling points can occur if nucleation sites are lacking – even two reactions apparently refluxing in the same solvent can be at different temperatures! In fact, simply getting the temperature wrong is probably the major reason for the disparate results obtained when conventional reactions are compared to their microwave ‘equivalents’. This isn't helped by the fact that your average lab microwave only reads the reaction temperature by IR measurements of the surface of the reaction vessel; I’ve heard descriptions of this practice ranging from ‘optimistic’ to ‘demonstrably hopelessly inaccurate’.

I'm reusing this photo of a microwave just to break up the text a bit!
Because of these (and various other) hard-to-isolate factors, it’s actually pretty hard to properly compare conventional and microwave heated reactions, and not everyone has the kit required to do so properly. This has led to numerous claims of so called ‘non-thermal’ or ‘specific’ microwave effects in the literature. These generally explain the apparent benefits of microwave heating by claiming that the microwaves don’t just simply heat the reaction medium (hence ‘non-thermal’), but instead excite (or even stabilise!) particular bonds or intermediates directly, in a fashion distinct from simple macroscopic heating of the reaction mixture. Such claims have been debunked for over a decade, and physical chemists will tell you—at least in the liquid phase—that energy is redistributed amongst the molecules in the reaction vessel on a much shorter timescale than the period of the microwaves used to excite them, making specific heating of one kind of molecule over another unlikely. Certainly, temperature gradients and macroscopic hotspots may well exist (particularly in viscous/high dielectric/inadequately stirred media), and are readily measured with a temperature probe, but I’ve yet to see credible evidence for the molecular-scale thermal aberrations that are continually reported. It seems that, when investigated in detail, with care to eliminate other factors, claims of non-thermal effects have yet to stand up to scrutiny. In fact, I'm a little baffled as to why we see the continued reporting of results predicated on this phenomenon, with few proper control experiments.
One of the most prominent chemists to voice their disbelief in so called ‘non-thermal microwave effects’ is Austrian Professor Oliver Kappe, who's been countering such claims in the literature for at least as long as I’ve been a chemist. He periodically publishes smack-downs of claims of chemistry of this type, most recently in an Angewandte Chemie Essay published just before Christmas (which I blogged about at the time). One of the groups whose work was criticised in this publication was that of Gregory Dudley at Florida state university, and he’s just hit back with a reply to Kappe’s earlier arguments, which has been met with a further rebuttal by Kappe. The last ‘literature boxing match’ of this type that I can recall was the citalopram back-and-forth in OPRD a couple of years back, covered at the time by Derek over at In The Pipeline, and while the claims made by either side here are not in the same league of dubiosity there’s plenty of thinly veiled frustration and strained civility to enjoy!
The Original Dudley Paper (2012)
Dudley’s original paper described an unusual Friedel-Crafts alkylation which Chemistry World rather incautiously covered in an article titled Magical Microwaves.[1] As you can see, it appears that the reaction does work better in the microwave, even at the same temperature:
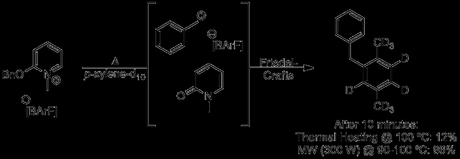
“Actually, it seems that Dudley and co-workers were quite careful, using the same volume reaction vessels for both runs, employing careful magnetic stirring and ensuring as much similarity as possible between the oil bath and microwave heated reactions. Unfortunately, despite its widely known limitations, the group chose to rely on (calibrated) IR sensors to monitor the temperature of the microwave reactions, arguing that in this case the low viscosity, well-stirred, homogenous nature of their reaction medium would allow accurate measurements to be taken.”
To be fair, Dudley did discuss this issue in depth, and also attempted to make a fair comparison by comparing microwave and conventionally heated reactions at reflux in open systems to ensure that any effects due to errors in temperature measurement could be isolated. They then larded the paper with a few asking-for-it phrases like 'clear benefit', 'rational design' and 'new paradigm' before sending it off for publication.
Kappe’s First Essay (2012)
To cut a long story short (see my earlier post for graphs), the Dudley group’s assumption on the accuracy of IR temperature sensors was a poor one. When Kappe re-ran some of Dudley’s reactions with a Fibre Optic (FO) temperature probe, they got near enough identical results for conventional and microwave reactions, concluding that erroneous temperature measurement was essentially the cause of Dudley’s apparent microwave enhancement:
“We have repeated the key experiments described by Dudley and co-workers, duplicating the reaction conditions and experimental setups reported in the original reference. The only difference is that in our experiments the reaction temperature in both the microwave and oil-bath runs was carefully monitored by accurate and fast responding internal FO probes…. Since our data sets more closely correspond to the conversions reported by Dudley and co-workers for their oil-bath experiments, we assume that the actual reaction temperatures in their microwave runs must have been significantly higher than the values recorded using external IR sensor technology. This hypothesis is also supported by the fact that the applied average microwave power in the Dudley experiments is considerably higher than the values in our experiments, even though the same type of microwave reactor was used.”
Dudley’s Reply (2013)
I honestly never though that Dudley would wade in to defend his work, as it seemed to me (a non-expert in microwave chemistry) that he’d just been a bit sloppy. However, he recently did, stating three main grievances with Kappe’s essay (the titles are Dudley’s):
1. Our Conclusions Are Misstated in the Essay and Their Conclusions Are Inconsistent
Dudley accuses Kappe of straw-man tactics; that is, misrepresenting his position and then attacking the misrepresentation. This is based around the fact that Kappe uses the term ‘non-thermal’ a total of 24 times in his Essay (and its SI) whereas Dudley doesn’t actually use the term in his original paper.
The second part of this complaint refers to the fact that in his first Essay Kappe wrote that the "key to achieving comparable conditions in the two types of experiments was the use of boiling chips that prevent superheating under microwave conditions" and ascribed most of Dudley’s microwave enhancement to ‘solvent superheating’. Dudley’s reply argues that Kappe’s use of the term ‘solvent superheating’ contradicts his claim that there are no non-thermal/specific microwave effects; i.e. Dudley seems to say that ‘solvent superheating’ is a non-thermal/specific microwave effect. I am not sure how I feel about this.
2. Our Experimental Conditions Were Not Appropriately Replicated
Briefly, Dudley’s major grievance here is he used a constant power of 300 W on his microwave (measuring temperatures <100 ºC), whereas Kappe used a much lower power but kept the reaction at an accurately measured, constant temperature of 100 ºC. Dudley argues that, as the microwaves are enhancing the reaction in some way, power is very important. However, Kappe did address this deviation from Dudley's conditions in his Essay, saying that he didn’t need as much power as his set-up was more thermally efficient, and temperature is much more important anyway. Essentially, Dudley contends that Kappe used blatantly different reaction conditions, invalidating his results.
3. The Text of the Essay Does Not Match the Supporting Information
As I mentioned above, in his original paper Dudley did perform a comparison of conventional and microwave heated reactions where the temperature was 'fixed' at 110 ºC by carrying out the reaction in refluxing toluene (to ‘rule out’ measurement errors from the IR sensor). Thus, identical reactions were run at reflux as obtained by conventional heating and reflux as obtained by microwave heating. Microwave heating gave much better results and, at first glance, this does appear to indicate some specific microwave effect as temperature and pressure should be the same for both reactions. Kappe repeated these experiments (the data’s in the Essay’s SI) and initially obtained what looked like the same results as Dudley, before adding boiling stones to decrease the yield of the microwave heated reaction to that of the conventionally heated one.
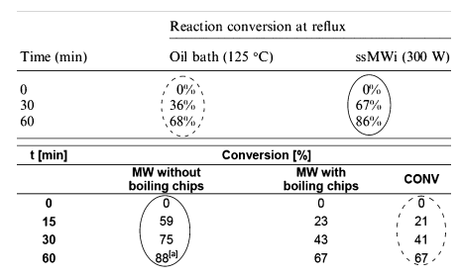
Figure 2 from Dudley's Letter. On the top, Dudley's original results. On the bottom, Kappe's repeat from his Essay. Yes, the numbers look similar, but as the addition of boiling chips shows, the microwave enhancement appears to be a superheating effect.
As Dudley see it: "Later in the Essay, the authors note, “Key to achieving comparable conditions…was the use of boiling chips that prevent superheating under microwave conditions” . In other words, they altered our experimental design—by adding boiling chips—explicitly to prevent what they describe as “a specific microwave effect”. It is incorrect for them to conclude that, “in our hands no evidence for any type of specific or non-thermal microwave effect could be obtained.” In fact, they did obtain such evidence. They just did not present the evidence in the main text of the Essay."
I'm not sure that implying these results were hidden is really fair, as there's a limit to what you can fit a two-page essay; the SI is some 24 pages long, so lots of other stuff, including more discussion, got bumped over. Furthermore, Dudley's original paper makes mention of specific, selective microwave excitation of that very polar starting material as a deliberate, rational design feature. It seems to me that it is largely this point that Kappe took issue with in his Essay. However, rather than defend his original assertion concerning selective 'microwave actuation', Dudley now seems to be changing the subject to superheating, a word that only appears twice in his first paper, and then only the footnotes where it's ruled out. Kappe deliberately removed superheating from the equation in order to probe Dudley's dubious 'microwave-actuation' claims, and should be credited for thoroughness. Dudley should have really have done this himself, rather than—like he did for the possibility of inaccurate temperature measurement—just dismiss it as unlikely! To be fair, it's not very clear in Kappe's Essay whether he regards superheating as a 'specific microwave effect' or not, but that's not really central to the argument – surely superheating is a thermal phenomenon?
The letter continues with a further discussion of superheating (a term Dudley doesn't like very much), and finishes with the following conclusion:
“Our original objective was to demonstrate an effect: that different chemical behaviors can be observed from otherwise identical chemical systems depending on whether conventional or microwave heating is employed. In this regard our results are clear: MW energy is an important variable in promoting thermal reactivity in the specific system that we studied… In the particular case of our paper, which unambiguously discussed and experimentally addressed the temperature problem, Prof. Kappe's conceit that we were somehow blithely ignorant of the problems of external IR thermometers is, to say the least, disingenuous… The fact is, microwaves heat in a manner that is intrinsically different from convective heating, and mechanisms exist, consistent with the fundamental physics of microwave heating, by which a “specific microwave effect” can potentially manifest itself. This effect is thermal in nature and arises from the selective heating of molecules in solution… Prof. Kappe's attempt to “debunk” our effort is without merit, as he neither accurately duplicated our deliberately chosen experimental conditions nor accurately determined the temperature in our refluxing solution.”
Finally, Dudley says rather forthrightly that Kappe probably set out to debunk rather than reproduce, with a non-objective mindset not in the interests of science, and that he firmly believes that the ‘alternative heating properties’ of microwaves can be exploited. You should probably check it out for yourself, but I can't help feel ing that Dudley's shifted position somewhat in this letter. The conclusion to Dudley's original paper clearly states that "the microwave-actuated reactivity of polar substrates in nonpolar solvents can exceed temperature-based expectations... The Friedel–Crafts benzylation reaction described herein provides a compelling example in which MW energy is an important variable for achieving the desired reactivity" and most people have interpreted this to mean non-thermal effects. Certainly, the author of the Magic Microwaves article on this work in Chemistry World, which is full of quotes from Dudley—and presumably okayed by him—understood this to be the case, but in this recent letter he now seems to be arguing that decidedly thermal effects like superheating are the real explanation.
Kappe’s Final Say (2013)
Kappe’s reply to Dudley’s comeback addresses the same three points, although I definitely get the impression he has a lot more to say on the matter:
1. Our Conclusions Are Misstated in the Essay and Their Conclusions Are Inconsistent
Kappe argues that firstly, only two—highly qualified—instances of ‘non-thermal’ are use in reference to the original Dudley paper (the other 22 are because Kappe’s Essay discusses several pieces of work) and, secondly, Dudley never really qualifies whether he thinks the microwave enhancement he observes is thermal/specific/non-thermal in nature anyway. He calmly states that whatever terminology is used, Dudley is probably seeing the results of solvent superheating under atmospheric conditions; inaccurate temperature measurement is the cause of the 'enhancement'.
2. Our Experimental Conditions Were Not Appropriately Replicated
Kappe is quite dismissive here, stating that power ratings on microwaves are nominal, instrument specific and not really comparable; hence the reason that he has argued for years that all microwave chemistry should report temperatures, not just powers.[2] Furthermore, he states that many solvents reflect the vast majority of microwave radiation anyway (for acetonitrile this is >95%, for hexane >99%; I guess toluene is somewhere in the middle), meaning that interpretation of the power rating on microwaves is ‘problematic’ at best. If Dudley measured temperature of <100 ºC when the reaction occurred, then surely performing it at 100 ºC should give it a fair chance as a similar amount of power must be absorbed in each case.
3. The Text of the Essay Does Not Match the Supporting Information
It turns out that there’s no big cover-up going on here; Kappe found that in the microwave reflux experiment without boiling stones, the reaction temperature (as accurately measured by fiber optic probe) was around 10 ºC higher, and stated this in his Essay! The stones were only added to put the microwave and conventional reactions on an equal footing thermally, and that’s why the un-stoned reaction data wasn’t explicitly given in the main text of the Essay. All of this is explained clearly in Kappe’s SI and he seems to resent Dudley’s allegations that this information was somehow hidden, saying "the discussion provided in the Supporting Information is an integral part of the Essay and cannot be considered separately from the main body of the Essay".[3]
There’s a bit more of a discussion on superheating, and Kappe reiterates that Dudley’s results are simply caused by this rather-hard-to-isolate phenomenon, which is , in essence, a thermal effect. He finishes with two fantastic broadsides, first saying that “We note that in their Correspondence, Dudley and co-workers did not respond to some of our general criticism of their experimental work, in particular the questionable NMR analysis, lack of statistical analysis of data, and some rather difficult to rationalize microwave hardware issues. For example, while the authors were able to heat a 2 mL reaction mixture to 80ºC using 88 W of average microwave power, they were unable to heat the same reaction mixture on the same instrument to 100ºC using 200 W or 300 W magnetron output power. This is rather difficult to comprehend.”
And then, for good measure, he questions the actual point of the work itself: “As a final thought we would like to point out that regardless whether the observed microwave effect in this controversial Friedel–Crafts alkylation discussed herein is due to selective reactant heating or bulk solvent superheating, both types of specific microwave effects are of little practical relevance to synthetic organic chemists…In the case of the putative selective reactant heating as proposed by Dudley and co-workers, it is hard to imagine that the strict design requirements (microwave transparent solvent, soluble but highly microwave-absorbing reactant, no other microwave-absorbing substrates, etc.) will allow a lot of flexibility or provide some unique selectivity in synthetic transformations that could not be achieved in a different way, for example by simply running the experiment at a higher bulk reaction temperature.”
The tone of Kappe’s Essay, and especially the two letters it spawned, is quite unusual for scientific papers, and I can’t fully convey it here, so I’d definitely recommend checking out the exchange yourself (and forming your own opinion).[4] Take care to read the footnotes as you go, as some of them are remarkably cutting. I have very little interest in microwave chemistry except, but I found this debate between two intelligent, articulate and well-respected scientists to be quite engrossing. As a non-linguist I am also in awe of the fact that Kappe can argue so persuasively in what I assume is his second language!
Etc
- I should point out that they’ve previously also covered Kappe’s side of things in the articles Microwaving Myths (not free) and the rather final-sounding Microwave Effect Ruled Out (free).
- I've had enough failed attempts to repeat procedures for microwave reactions that only give power to agree with this – it just doesn't make for reproducible chemistry. If the power is fixed, but the reaction is scaled up from the reported procedure and solvent volume (or any other variable) is changed then a different temperature will result. As temperature—for the time being—seems to be the major factor influencing reaction outcome in a microwave reactor, this inevitably affects the results. The worst kind are the old, domestic microwave procedures along the lines of "microwave in a beaker on 'High' for 15-60 mins".
- That’s interesting, as Angewandte explicitly states that SI is not peer reviewed, for one thing…
- If you don't have access to Angewandte, some kind soul's put the papers up on Reddit, where discussion's been going on for a little while.
