CC Chow, KK Finn, GB Storchan, X Lu, X Sheng, SS Simons Jr., Kinetically-Defined Component Actions in Gene Repression. PLoS Comp Bio. 11:e1004122, (2015)
Abstract
Gene repression by transcription factors, and glucocorticoid receptors (GR) in particular, is a critical, but poorly understood, physiological response. Among the many unresolved questions is the difference between GR regulated induction and repression, and whether transcription cofactor action is the same in both. Because activity classifications based on changes in gene product level are mechanistically uninformative, we present a theory for gene repression in which the mechanisms of factor action are defined kinetically and are consistent for both gene repression and induction. The theory is generally applicable and amenable to predictions if the dose-response curve for gene repression is non-cooperative with a unit Hill coefficient, which is observed for GR-regulated repression of AP1LUC reporter induction by phorbol myristate acetate. The theory predicts the mechanism of GR and cofactors, and where they act with respect to each other, based on how each cofactor alters the plots of various kinetic parameters vs. cofactor. We show that the kinetically-defined mechanism of action of each of four factors (reporter gene, p160 coactivator TIF2, and two pharmaceuticals [NU6027 and phenanthroline]) is the same in GR-regulated repression and induction. What differs is the position of GR action. This insight should simplify clinical efforts to differentially modulate factor actions in gene induction vs. gene repression.
Author Summary
While the initial steps in steroid-regulated gene induction and repression are known to be identical, the same cannot be said of cofactors that modulate steroid-regulated gene activity. We describe the conditions under which a theoretical model for gene repression reveals the kinetically-defined mechanism and relative position of cofactor action. This theory has been validated by experimental results with glucocorticoid receptors. The mode and position of action of four factors is qualitatively identical in gene repression to that previously found in gene induction. What changes is the position of GR action. Therefore, we predict that the same kinetically-defined mechanism usually will be utilized by cofactors in both induction and repression pathways. This insight and simplification should facilitate clinical efforts to maximize desired outcomes in gene induction or repression.
I am so happy that this paper is finally published. It was a two-year ordeal from the time I had the idea of what to do until it finally came out. This is the second leg of the three-legged stool for a theory of steroid-regulated gene expression. The first was developing the theory for gene induction (e.g. see here) that started over ten years ago when Stoney and I first talked about trying to understand his data and really took off when Karen Ong turned her summer internship into a two-year baccalaureate fellowship. She’s now finishing up the PhD part of her MD-PhD at the Courant Institute at NYU.
In the first leg, we showed that if the dose-response curve for steroid-regulated gene induction (i.e. gene product as a function of ligand concentration), had the form
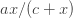
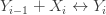
![[Y_n] [Y_n]](http://m5.paperblog.com/i/118/1181447/new-paper-on-gene-repression-L-tuFrWF.png)
![[Y_0] [Y_0]](http://m5.paperblog.com/i/118/1181447/new-paper-on-gene-repression-L-dtF4wd.png)
However, steroids also repress genes and interestingly enough the repression curve is also noncooperative and is given by the linear fractional function
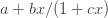
I’m currently putting on the finishing touches for revisions on the third leg of the stool now. We have even reunited the band and convinced Karen to take some time away from her thesis to help finish it. This paper is about how partial agonists or antagonists like tamoxifen work, which could have implications for drug development and avoiding side effects. Steroids are not the only ligand that can activate a steroid-regulated gene. The steroid cream that you use for rashes consists of a highly potent steroid agonist. There are also molecules that block or impede the action of steroids by binding to steroid receptors and these are called partial agonists, antagonists or antisteroids. However, steroid receptors are widely expressed and that is why when you take them they can have severe side effects. Hence, it would be nice to be able to control where they act and by how much. This third leg paper is the theory behind how to do this.
