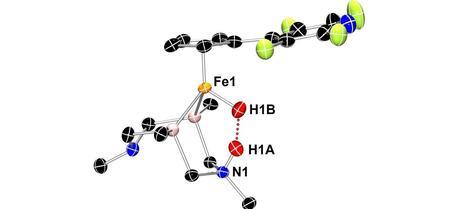
 Neutron crystallography shows this iron catalyst gripping two hydrogen atoms (red spheres). This arrangement allows an unusual dihydrogen bond to form between the hydrogen atoms (red dots). (Credit: PNNL)
Neutron crystallography shows this iron catalyst gripping two hydrogen atoms (red spheres). This arrangement allows an unusual dihydrogen bond to form between the hydrogen atoms (red dots). (Credit: PNNL)The first view of nature-inspired catalyst after ripping hydrogen apart provides insights for better, cheaper fuel cells.
This study is the first time scientists have shown precisely where the hydrogen halves end up in the structure of a molecular catalyst that breaks down hydrogen, the team reported online April 22 in Angewandte Chemie International Edition (see footnote). The design of this catalyst was inspired by the innards of a natural protein called a hydrogenase enzyme.
“The catalyst shows us what likely happens in the natural hydrogenase system,” said Morris Bullock of the Department of Energy’s Pacific Northwest National Laboratory. “The catalyst is where the action is, but the natural enzyme has a huge protein surrounding the catalytic site. It would be hard to see what we have seen with our catalyst because of the complexity of the protein.”
Hydrogen-powered fuel cells offer an alternative to burning fossil fuels, which generates greenhouse gases. Molecular hydrogen—two hydrogen atoms linked by an energy-rich chemical bond—feeds a fuel cell. Generating electricity through chemical reactions, the fuel cell spits out water and power.
If renewable power is used to store energy in molecular hydrogen, these fuel cells can be carbon-neutral. But fuel cells aren’t cheap enough for everyday use.
To make fuel cells less expensive, researchers turned to natural hydrogenase enzymes for inspiration. These enzymes break hydrogen for energy in the same way a fuel cell would. But while conventional fuel cell catalysts require expensive platinum, natural enzymes use cheap iron or nickel at their core.
Researchers have been designing catalysts inspired by hydrogenase cores and testing them. In this work, an important step in breaking a hydrogen molecule so the bond’s energy can be captured as electricity is to break the bond unevenly. Instead of producing two equal hydrogen atoms, this catalyst must produce a positively charged proton and a negatively charged hydride.
The physical shape of a catalyst—along with electrochemical information—can reveal how it does that. So far, scientists have determined the overall structure of catalysts with cheap metals using X-ray crystallography, but hydrogen atoms can’t be located accurately using X-rays. Based on chemistry and X-ray methods, researchers have a best guess for the position of hydrogen atoms, but imagination is no substitute for reality.
Bullock, Tianbiao “Leo” Liu and their colleagues at the Center for Molecular Electrocatalysis at PNNL, one of DOE’s Energy Frontier Research Centers, collaborated with scientists at the Spallation Neutron Source at Oak Ridge National Laboratory in Tennessee to find the lurking proton and hydride. Using a beam of neutrons like a flashlight allows researchers to pinpoint the nucleus of atoms that form the backbone architecture of their iron-based catalyst.
To use their iron-based catalyst in neutron crystallography, the team had to modify it chemically so it would react with the hydrogen molecule in just the right way. Neutron crystallography also requires larger crystals as starting material compared to X-ray crystallography.
“We were designing a molecule that represented an intermediate in the chemical reaction, and it required special experimental techniques,” Liu said. “It took more than six months to find the right conditions to grow large single crystals suitable for neutron diffraction. And another six months to pinpoint the position of the split H2 molecule.”
Crystallizing their catalyst of interest into a nugget almost 40 times the size needed for X-rays, the team succeeded in determining the structure of the iron-based catalyst.
The structure, they found, confirmed theories based on chemical analyses. For example, the barbell-shaped hydrogen molecule snuggles into the catalyst core. On being split, the negatively charged hydride attaches to the iron at the center of the catalyst; meanwhile, the positively charged proton attaches to a nitrogen atom across the catalytic core. The researchers expected this set-up, but no one had accurately characterized it in an actual structure before.
In this form, the hydride and proton form a type of bond uncommonly seen by scientists—a dihydrogen bond. The energy-rich chemical bond between two hydrogen atoms in a molecule is called a covalent bond and is very strong. Another bond called a “hydrogen bond” is a weak one formed between a slightly positive hydrogen and another, slightly negative atom.
Hydrogen bonds stabilize the structure of molecules by tacking down chains as they fold over within a molecule or between two independent molecules. Hydrogen bonds are also key to water surface tension, ice’s ability to float and even a snowflake’s shape.
The dihydrogen bond seen in the structure is much stronger than a single hydrogen bond. Measuring the distance between atoms reveals how tight the bond is. The team found that the dihydrogen bond was much shorter than typical hydrogen bonds but longer than typical covalent bonds. In fact, the dihydrogen bond is the shortest of its type so far identified, the researchers report.
This unusually strong dihydrogen bond likely plays into how well the catalyst balances tearing the hydrogen molecule apart and putting it back together. This balance allows the catalyst to work efficiently.
“We’re not too far from acceptable with its efficiency,” said Bullock. “Now we just want to make it a little more efficient and faster.”
Liu, T., Wang, X., Hoffmann, C., DuBois, D., & Bullock, R. (2014). Heterolytic Cleavage of Hydrogen by an Iron Hydrogenase Model: An Fe-H⋅⋅⋅H-N Dihydrogen Bond Characterized by Neutron Diffraction Angewandte Chemie DOI: 10.1002/ange.201402090
