
Three of the coral species studied by Muir (2): (a) Acropora pichoni: Pohnpei Island, Pacific Ocean — deep-water species/IUCN ‘Near threatened’; (b) Acropora divaricate: Maldives, Indian ocean — mid-water species/IUCN ‘Near threatened’; and (c) Acropora gemmifera: Orpheus Island, Australia — shallow-water species/IUCN ‘Least Concern’. The IUCN states that the 3 species are vulnerable to climate change (acidification, temperature extremes) and demographic booms of the invading predator, the crown-of-thorns starfish Acanthaster planci. Photos courtesy of Paul Muir.
Global warming of the atmosphere and the oceans is modifying the distribution of many plants and animals. However, marine species are bound to face non-thermal barriers that might preclude their dispersal over wide stretches of the sea. Sunlight is one of those invisible obstacles for corals from the Indian and Pacific Oceans.
If we were offered a sumptuous job overseas, our professional success in an unknown place could be limited by factors like cultural or linguistic differences that have nothing to do with our work experience or expertise. If we translate this situation into biodiversity terms, one of the best-documented effects of global warming is the gradual dispersal of species tracking their native temperatures from the tropics to the poles (1). However, as dispersal progresses, many species encounter environmental barriers that are not physical (e.g., a high mountain or a wide river), and whose magnitude could be unrelated to ambient temperatures. Such invisible obstacles can prevent the establishment of pioneer populations away from the source.
Corals are ideal organisms to study this phenomenon because their life cycle is tightly geared to multiple environmental drivers (see ReefBase: Global Information System for Coral Reefs). Indeed, the growth of a coral’s exoskeleton relies on symbiotic zooxanthellae (see video and presentation), a kind of microscopic algae (Dinoflagellata) whose photosynthetic activity is regulated by sea temperature, photoperiod and dissolved calcium in the form of aragonite, among other factors.
Along those lines, Paul Muir and collaborators collated depth and latitude in the Indian and Pacific oceans for more than 14 thousand records of Acropora and Isopora species (2) – collectively known as ‘staghorn corals’ for their resemblance to deer antlers. Members of the family Acroporidae include over 140 species of the so-called hard or stony corals (Scleractinia), known to the primitive Mediterranean in the Triassic, which makes them > 200 million-year old (3). In particular, the genus Acropora originated some 50 million years ago and today populates all major oceans (4).
Muir found that most tropical records of Acropora and Isopora from the Equator to 25 degrees latitude (north or south) are from depths below 24 metres. In contrast, most temperate records (beyond ~ 25 degrees latitude) rarely exceed depths of 11 metres. And when comparing the biogeography of these corals with the horizontal and bathymetric distribution of several environmental factors (aragonite, sunlight, salinity, temperature), Muir could best reconstruct coral bathymetric boundaries using the amount of sunlight available for photosynthesis as predictor.
The emerging pattern is that deep-living habits in corals are restricted to the tropics and vanish gradually north and south of 20 to 25 degrees latitude — and the pattern holds for depth specialists as well as for species tolerant to wide depth profiles (2). Essentially, sunlight seems to limit coral growth (mediated by zooxanthella primary production) even in optimal conditions of aragonite, salinity and temperature.
To date, climate-change studies on corals have mostly focused on ocean acidification and bleaching events (5, 6). Acidification, due to high concentrations of dissolved carbon dioxide, decreases seawater pH and can block calcium assimilation and/or dissolve the exoskeleton of corals. Bleaching, due to high (and sometimes low) seawater temperature, can force the expulsion of zooxanthella, after which corals whiten and often experience massive die-offs due to photosynthetic impairment. In the tropics where most corals live, these invertebrates could escape from seawater warming by moving their distributions toward the Equator, as has been detected in Japan (7).
Nevertheless, Muir’s study demonstrates that the expansion of the distribution of staghorn corals also depends on the interaction between sunlight and depth. If you were a coral from shallow waters dispersing towards the temperate zone, you would have to counteract increased wave strength, salinity, resource competition with temperate macroalgae and corals, and often air exposure during tides. On top of that, if you were a deep-water coral species, you would face a light deficit — all the more enhanced by the loss of water transparency owing to human disturbance of coastal waters (e.g., dredging, eutrophication, chemical and solid wastes).
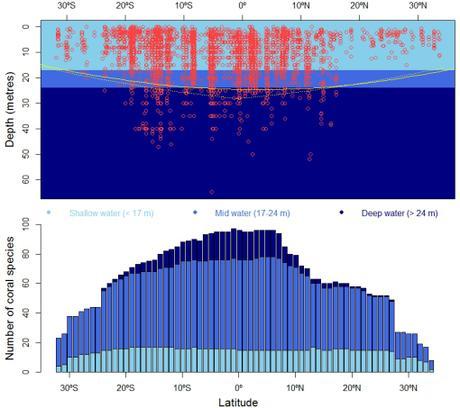
Records of 104 species of staghorn corals (Acropora & Isopora) in the Indian and Pacific Oceans, some 35 degrees north and south of the Equator (abscissa) — given contrasting adaptation of species to shallow, mid and deep waters (2). The upper panel shows the increase in deep-water records (red circles) from temperate (~ ±35º latitude) to Equatorial (0° latitude) waters — the continuous yellow line captures the overall latitudinal trend and the dashed line is the prediction of such trend using the amount of light available for photosynthesis (i.e., the key factor allowing symbiont zooxanthella to provide the basal metabolic requirements of their hosts). The lower panel shows the frequency of coral species at three depth intervals; clearly, from the temperate zone to the Equator, species richness increases mainly due to mid- and deep-water species, the latter being bounded between ~ ±25º latitude. For those readers unfamiliar with the Indian and Pacific Oceans, by analogy the study region would approximately comprise the Atlantic expanse between Madagascar and the Canary Islands.
The bad news is that staghorn corals might be environmentally cornered in their tropical homes, so they must either evolve or perish. Not only that, because staghorn corals (i) have become the most successful reef-building species through geological time (due to high growth rates and propagation by fragmentation), but (ii) are highly susceptible to environmental stress, their ongoing global impoverishment could lead to coral-reef ecosystem collapse altogether (8).
It is a gloomy future for corals indeed (9), and it comes as no surprise that because traditional conservation and management practices cannot keep pace with anthropogenic climate change combined with multiple additional stressors, recent research advocates for the artificial selection for coral varieties endowed with enhanced resilience to stress (‘assisted evolution’; 10, 11) — as if they were orchard plantations!
—
by Salvador Herrando-Pérez & David R. Vieites
Museo Nacional de Ciencias Naturales-CSIC. @vieites, @hp_salva. Funded by the British Ecological Society and the Spanish Ministry of Economy, Industry and Competitiveness.
—
References
- Pecl, GT et al. (2017) Biodiversity redistribution under climate change: impacts on ecosystems and human well-being. Science 355: eaai9214
- Muir, PR et al. (2015) Limited scope for latitudinal extension of reef corals. Science 348: 1135-1138
- Stanley Jr, GD (2003) The evolution of modern corals and their early history. Earth-Science Reviews 60: 195-225
- Wallace, CC, Rosen BR (2006) Diverse staghorn corals (Acropora) in high-latitude Eocene assemblages: implications for the evolution of modern diversity patterns of reef corals. Proceedings of the Royal Society B: Biological Sciences 273: 975-982
- Doney, SC et al. (2012) Climate change impacts on marine ecosystems. Annual Review of Marine Science 4: 11-37
- Hoegh-Guldberg, O et al. (2007) Coral reefs under rapid climate change and ocean acidification. Science 318: 1737-1742
- Yamano, H et al. (2011) Rapid poleward range expansion of tropical reef corals in response to rising sea surface temperatures. Geophysical Research Letters 38: L04601
- Renema, W et al. (2016) Are coral reefs victims of their own past success? Science Advances 2: e1500850
- Jackson, JBC (2008) Ecological extinction and evolution in the brave new ocean. Proceedings of the National Academy of Sciences of the USA 105: 11458-11465
- van Oppen, MJH et al. (2015) Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences 112: 2307-2313
- van Oppen, MJH et al. (2017) Shifting paradigms in restoration of the world’s coral reefs. Global Change Biology doi:10.1111/gcb.13647

