An interesting paper appeared in Angewandte Chemie yesterday detailing a re-investigation of a number of mercury azides that were—for reasons that will become apparent—not properly characterized when they were first reported in the literature back in the 1890s.[1] This publication is remarkable in a number of ways, not least that it has made today’s report on (trinitromethyl)borate synthesis seem rather boring and jejune in comparison.
"Always look on the bright azide of life" —Image and pun from Angewandte Chemie, 2013, Early View
It turns out that Hg2(N3)2 and α-Hg(N3)2 are both easily prepared using reported methods and are display predictable instability/toxicity, but nothing to write home about. The most exciting part of this paper focuses on the alternative β- form of Hg(N3)2. The authors describe the procedure as follows:
“In analogy to the preparation of β-Pb(N3)2, a second metastable modification of mercury(II) azide, β-Hg(N3)2 can be obtained by slow diffusion of aqueous NaN3 into a solution of mercury(II) nitrate which is separated by a layer of aqueous NaNO3. Thereby, needle-like crystals of β-Hg(N3)2 start to form in the lower mercury(II) nitrate layer which is always accompanied by spontaneous explosions during crystal growth finally leading to a mixing of the layers and the fast precipitation of α-Hg(N3)… Slow crystallization during the preparation of α- or β-Hg(N3)2 leads to the formation of large crystals which are extraordinarily sensitive to all kinds of provocation (e.g. even detonate in solution) and therefore should be avoided by all means. Nevertheless, with extreme care, we were able to manually isolate some specimens of β-Hg(N3)2 under the microscope which allowed the characterization by vibrational spectroscopy, single-crystal X-ray diffraction, and the determination of the melting point.”
Now, when people talk about metastability I think of things like diamond and Dewar benzene; substances that actually have an appreciable energy barrier to their decay. You know, the sort of thing where you can say “hey, check this out! It’s metastable!” without your statement being punctuated by detonations and the sound of breaking glass followed by screams. Seriously, how are you supposed to prepare a compound that detonates at random under its own weight during crystallisation?
That said, if you look at the detailed procedure for the synthesis of β- Hg(N3)2 in the paper’s supporting information and skip the line that cautions “during this period explosions frequently occur” (just keep calm and carry on), then once you’ve made and isolated the compound it does sound surprisingly stable. In fact, once dry and pure—and after some rather fraught measurements by one of the students—the group was able to determine that the compound was stable up to 180 ºC when it sublimed. One day, I would like to meet the kind of person that works on projects like this!
- Of course, charactization during that period largely revolved around melting point, taste and combustion analysis, all of which are hugely inappropriate for explosive mercury compounds (although I don’t doubt that people tried; the Merck index includes information on the taste of pyridine, presumably obtained shortly after its isolation a few decades previously).
- Also, does this figure from the paper seem a bit strange to you?
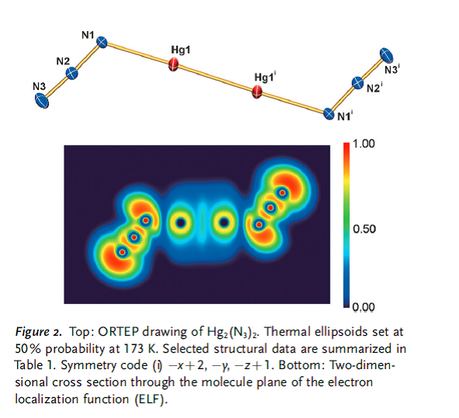
Alternative caption: Figure 2. Top: ORTEP drawing of Hg2(N3)2.
Thermal ellipsoids set at 50% probability at 173 K. Selected
structural data are summarized in Table 1. Symmetry code (i) x+2, y, z+1.
Bottom: Owl in flight, seen during acid trip.
