Heat and work; a simple yet complex thermodynamics topic. Most students have misconceptions about the concept of heat and work in thermodynamics. You may have the following questions in mind and looking for answers:
- What is heat and work?
- Are heat and work forms of energy?
- What is the difference between heat and work?
If you’re looking for answers to the questions above, then you’re at the right place. In order to provide a clear concept of heat and work to you, we’ve covered all aspects of the topic in this article.
Heat and work are two dissimilar ways of transferring energy from one system to another. Both heat and work have the same unit of measurement J (joule) and thus most students think that work is energy.

Let’s see if that’s right! We can define ‘work’ as a mode which transfer mechanical energy between two systems. It means work is only a medium or a method which carries energy from one system to another. For example, Spiderman applies a force on a moving truck to prevent it from moving. This is an example of work being done. Spiderman transfers energy to the truck to stop it via a medium that we call ‘work’.
In contrast, Heat is the transfer of thermal energy between systems. Work can be transformed into heat and vice versa. It’s essential to differentiate between the macroscopic motion (work) and microscopic (heat) and is vital to how thermodynamic processes work.
Difference Between Work and Heat – A Microscopic Interpretation
We will analyze the two concepts further and express their differences at both macroscopic & microscopic levels. At macroscopic level there is no difference between work and heat and they are both defined as modes which transfer energy (thermal or mechanical) from one object to another. But when we come to analyze these two terms, at microscopic level, things get a little bit changed but still simple. Physicists have defined ‘work’ in physics; a measure of energy transfer that occurs when an object is moved over a distance by an external force. It means, we have transferred energy to an object while changing its position by means of doing work on the object.
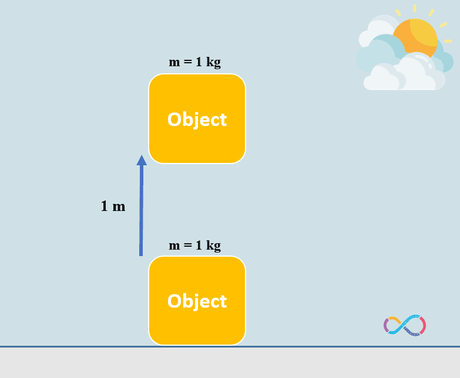
The equation to get the potential energy transferred to a displaced object is PE = mgh where; m stands for mass of the object (kg), g stands for the gravity which is 9.81 m/s2 and h is the height or distance which the object is disturbed to change its position. The illustration below shows an object with a mass of 1 kg moved over a vertical distance of 1 m.
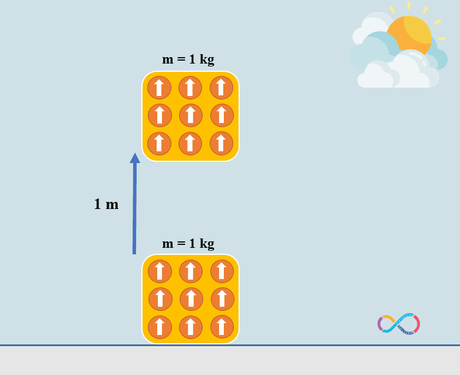
In microscopic level we define work as ordered motion of particles which means that all molecules of the object are pointing to the same direction while transferring energy to the object when we do work on it and this is why work results in “high-quality” energy. In contrast, heat is the random and unordered motion of particles and thus it results in “low-quality” energy. When we add some energy to an object by means of heat (increasing its temperature) the molecules of the object are no longer pointing to the same direction and they make random and disordered motion, and this random motion differs heat from work in microscopic level.
Let’s consider an example to know the concepts further. If we have an object with a mass of 1 kg and the object is displaced by 1 m. The energy transferred to the object by means of work can be determined as: mgh = 1 x 1 x 9.81 = 9.81 J or approximately 10 J. This means that if we have an object with a mass of 1 kg and we do some work on the object to displace it by 1 m, in fact we have transferred 10 joules of energy to the object as shown in the illustration below.
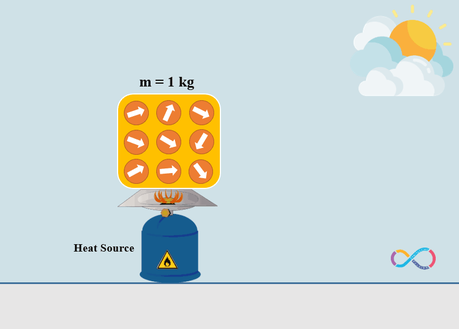
Consider that same amount of energy (10 J) is transferred to an object but this time by means of heat. As shown in the illustration below, the molecules of the object are heated by the gas cylinder (heat source) and the molecules of the object are pointing in random directions and are making an unordered motion.
Summary:
When you do work on an object, some of your energy is transferred to the object. You can think of work as the transfer of mechanical energy. Heat is the transfer of thermal energy. Work requires force and displacement and heat requires temperature difference. In fact, work, heat and energy are measured in the same unit, the joule. However, heat and work are not considered energy or forms of energy. Heat and work are only methods of energy transfer. In microscopic level, work results in ordered motion of particles while heat results in random and disordered motion of particles.
Here is a questions for you to answer: Can we store work or heat?
We hope that this simple explanation has clarified the concepts. Share your thoughts about the topic in the comment box.

