Many ecological studies have examined the tolerance of terrestrial wildlife to high and low air temperatures over global scales (e.g., 1, 2, 3). This topic has been boosted in the last two decades by ongoing and predicted impacts of climate change on biodiversity (see summary of 2019 United Nation’s report here and here).
However, it is unfortunate that for most species, studies have measured thermal tolerance from a single location or population. Researchers interested in global patterns of thermal stress collect those measurements from the literature for hundreds to thousands of species [recently compiled in the GlobTherm database] (4), and are therefore often restricted to analysing one value of thermal tolerance per species.

Three of the 15 species of Iberian lacertids sampled in our study of thermal tolerance (9), including the populations of Algerian psammodromus (Psammodromus algirus), Geniez’s wall lizard (Podarcis virescens) and Western green lizard (Lacerta bilineata) sampled in Navacerrada (Madrid), Fuertescusa (Cuenca) and Moncayo (Soria), respectively. Photos by S. Herrando-Pérez
Using this approach, ecologists have concluded that cold tolerance is far more variable than heat tolerance across species from the tropics to the boreal zone (5-8). Consequently, tolerance to heat stress might be a species trait with limited potential to change in response to global warming compared to cold tolerance (5).
In our recent study published in Functional Ecology, we quantified the heat and cold tolerance of 299 males from 58 different populations belonging to 15 species of Iberian lizards (Family Lacertidae) (you can download the full dataset from the Dryad repository). Thus, unlike global studies, we had measurements of thermal tolerance from two to five different populations (3 to 10 male lizards per population) within the spatial distribution of each study species.
We then followed three analytical steps: (i) randomly selected one population per species, (ii) tested for differences in the variability of heat versus hot tolerance across the 15 species using five (homoscedasticity) statistical tests (all of which gave identical results), and (iii) repeated the previous two steps thousands of times. This is akin to doing a study of thermal tolerance thousands of times, but (as implemented in global studies) each time using one unique population to represent the thermal tolerance of a species.
We measured a population’s heat and cold tolerance as the mean over all individuals sampled from that population. We estimated the heat and cold tolerance of each lizard as the temperature at which the individual [subject to increasing or decreasing temperatures, respectively] could not flip its body from a belly-up position (9, 10). Thus, the median heat and cold tolerances were 42.7 and 6.2 ºC over the 299 individuals sampled, respectively.
Our two main outcomes were compelling. Contrary to observed global patterns, we found that heat tolerance was more variable than cold tolerance six times more frequently than the opposite scenario among our thousands of hypothetical studies.
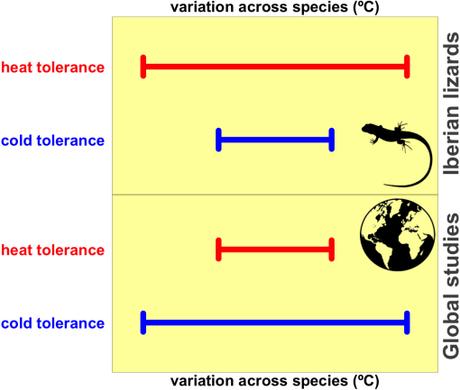
Graphical summary sketching the variation in heat and cold tolerance to high and low temperatures, respectively, in Iberian species of lacertid lizards [upper panel] versus global studies using hundreds to thousands of animal species [lower panel].
Moreover, depending on the population selected for each species, the variability of heat tolerance could outnumber 0 to 16 times the variability of cold tolerance across species. So, had we done a study sampling thermal tolerances from one population per species, the chances that we would have found heat tolerance to vary more than cold tolerance would have been much greater than the opposite scenario.
Our findings indicate that to capture the variation of thermal tolerance in the tree of life more comprehensively, we ideally need to measure it over the entire distribution of individual species.
While cold tolerance might be intimately linked to global gradients of chilling temperatures for terrestrial fauna, heat tolerance might respond to high temperatures at much smaller spatial scales. Such a response could go unnoticed in global studies and might help determine which species are likely to cope with warming.
This makes sense. Even though it is obvious that air temperatures rise (on average) along the gradient from tropical, temperate, boreal, to polar zones, climate change is multi-directional (11). Within a region, southern or western locations can therefore warm over time more (and more quickly) than northern or eastern locations, and vice versa (Australian example documented by 12).
Further, the daily thermal environment experienced by lizards can shift by up to 20ºC (13) as a result of landscape heterogeneity (vegetation, topography, geology). Such spatial variation in air temperatures compares well with the magnitude of warming expected in the most pessimistic scenarios of future climate change (14), which is bound to exert strong effects on the physiology of animals and plants and modify the magnitude of climate impacts (15) [see my blog here]. This phenomenon should be considered carefully in global ecology and to deal with global environmental changes.
Note 1: Salvador will be giving a seminar at Flinders University on 7 February 2020. See here for details.
Note 2: Field and lab work done by Camila Monasterio, Verónica Gomes and Wouter Beukema, with field support by Tim Leerschool, Filipe Serrano and Matthijs Hollanders. Funding provided by British Ecological Society grant (4496-5470) to me, and Spanish Ministry of Economy and Competitiveness (CGL2011-26852) and European Union (IC&DT 1/SAESCTN/ALENT-07-0224-FEDER-001755) projects to Miguel Bastos Araújo. This blog is a modified version of the plain summary published in Functional Ecology, and I dedicate it to my dad since I wrote it next to him in hospital while he was recovering from a health problem.
References
- C. A. Deutsch, J. J. Tewksbury, R. B. Huey, K. S. Sheldon, C. K. Ghalambor, D. C. Haak, P. R. Martin, Impacts of climate warming on terrestrial ectotherms across latitude. Proceedings of the National Academy of Sciences of the USA 105, 6668-6672 (2008)
- J. M. Sunday, A. E. Bates, N. K. Dulvy, Global analysis of thermal tolerance and latitude in ectotherms. Proceedings of the Royal Society B 278, 1823-1830 (2010)
- R. B. Huey, C. A. Deutsch, J. J. Tewksbury, L. J. Vitt, P. E. Hertz, H. J. Álvarez Pérez, T. Garland, Why tropical forest lizards are vulnerable to climate warming. Proceedings of the Royal Society B 276, 1939-1948 (2009)
- J. M. Bennett, P. Calosi, S. Clusella-Trullas, B. Martínez, J. Sunday, A. C. Algar, M. B. Araújo, B. A. Hawkins, S. Keith, I. Kühn, C. Rahbek, L. Rodríguez, A. Singer, F. Villalobos, M. Á. Olalla-Tárraga, I. Morales-Castilla, GlobTherm, a global database on thermal tolerances for aquatic and terrestrial organisms. Scientific Data 5, 180022 (2018)
- A. A. Hoffmann, S. L. Chown, S. Clusella-Trullas, Upper thermal limits in terrestrial ectotherms: how constrained are they? Functional Ecology 27, 934-949 (2013)
- J. W. Grigg, L. B. Buckley, Conservatism of lizard thermal tolerances and body temperatures across evolutionary history and geography. Biology Letters 9, 20121056 (2013)
- A. R. Gunderson, J. H. Stillman, Plasticity in thermal tolerance has limited potential to buffer ectotherms from global warming. Proceedings of the Royal Society B 282, 20150401 (2015)
- M. B. Araújo, F. Ferri-Yáñez, F. Bozinovic, P. A. Marquet, F. Valladares, S. L. Chown, Heat freezes niche evolution. Ecology Letters 16, 1206-1219 (2013)
- R. B. Huey, R. D. Stevenson, Integrating thermal physiology and ecology of ectotherms: A discussion of approaches. Integrative and Comparative Biology 19, 357-366 (1979)
- W. I. Lutterschmidt, V. H. Hutchison, The critical thermal maximum: History and critique. Canadian Journal of Zoology 75, 1561-1574 (1997)
- R. A. Garcia, M. Cabeza, C. Rahbek, M. B. Araújo, Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579 (2014)
- J. VanDerWal, H. T. Murphy, A. S. Kutt, G. C. Perkins, B. L. Bateman, J. J. Perry, A. E. Reside, Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nature Climate Change 3, 239-243 (2013)
- M. W. Sears, E. Raskin, J. M. J. Angilletta, The world is not flat: Defining relevant thermal landscapes in the context of climate change. Integrative and Comparative Biology 51, 666-675 (2011)
- A. J. Suggitt, P. K. Gillingham, J. K. Hill, B. Huntley, W. E. Kunin, D. B. Roy, C. D. Thomas, Habitat microclimates drive fine-scale variation in extreme temperatures. Oikos 120, 1-8 (2011)
- S. Herrando-Pérez, F. Ferri-Yáñez, C. Monasterio, W. Beukema, V. Gomes, J. Belliure, S. L. Chown, D. R. Vieites, M. B. Araújo, Intraspecific variation in lizard heat tolerance alters estimates of climate impact. Journal of Animal Ecology 88, 247-257 (2019)

