
Prof. Jong-Beam Baek (center) and his research team. (Credit: UNIST)
A group of scientists from the Ulsan National Institute of Science and Technology (UNIST), Korea, has developed a new metal-free fuel cell catalyst using edge-halogenated graphene nanoscale platelets. As a replacement for the expensive platinum-based catalysts this graphene application opens a way to affordable fuel cells.
The research team of Profs. Jong-Beam Baek and Noejung Park of UNIST, and Prof. Liming Dai of Case Western Reserve University, for the first time, reportedly synthesized a series of edge-selectively halogenated (Cl, Br and I) graphene nanoplatelets (XGnPs) by ball-milling graphite flake in the presence of chlorine (Cl2), bromine (Br2), or iodine (I2), respectively. The newly prepared XGnPs as metal-free electrocatalysts for oxygen reduction reaction (ORR) stand as a possible replacement for platinum (Pt), which is currently the most reliable material for cathodic ORR electrocatalysts in fuel cells.
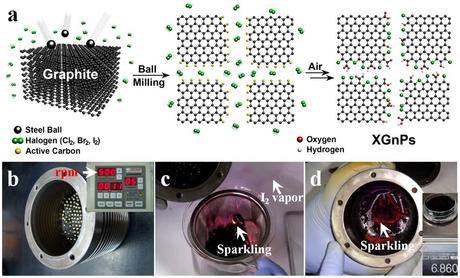
(a) A schematic representation for mechanochemically driven edge-halogenation reaction between the in-situ generated active carbon species (gold balls) and reactant halogens (twin green balls). Active carbon species were generated by homolytic bond cleavages of graphitic C-C bonds and reacted with halogen molecules to produce edge-halogenated graphene nanoplatelets (XGnPs) in a sealed ball-mill capsule and the remnant active carbon species are terminated upon subsequent exposure to air moisture. Red and gray balls stand for oxygen and hydrogen, respectively; (b) ball-mill capsule containing the pristine graphite and stainless steel balls (diameter 5 mm); (c) violent sparkling (red spots) occurred when the reaction mixture was exposed to ambient air moisture and excess purple I2 was sublimated in the air (arrow); (d) continued sparkling from residual IGnP at the bottom of a ball-mill capsule even after most of the IGnPs and stainless balls were taken out. (Credit: See citation at the end of this article)
Edge-halogenated graphene nanoplatelets (XGnPs) are solution processable, and show remarkable electrocatalytic activity toward ORR with a high selectivity, good tolerance and excellent long-term cycle stability.
One of the major drawbacks for commercialization of the fuel cell technology is the dragging ORR at cathode. So far, costly and precious Pt and its alloys have been considered to be the most reliable cathodic ORR electrocatalysts in fuel cells. However, Pt and its alloys have a drawback in that they suffer from methanol crossover/carbon monoxide (CO) poisoning effects and poor operation stability.
Although extensive efforts have been devoted to the development of non-precious metal-based electrocatalysts, their practical application is still far from being a reality due to their limited electrocatalytic activity, poor cycle stability, and sometimes environmental hazard.
Alternatively, carbon-based materials, doped with heteroatoms such as boron (B), halogen (Cl, Br, I) nitrogen (N), phosphorus (P), sulfur (S) and their mixtures, have attracted tremendous attentions as metal-free ORR electrocatalysts. However, full potential of these carbon-based, metal-free catalysts is hard to achieve without the synthetic capability for large-scale, low-cost production of the heteroatome-doped, carbon-based materials.
These novel metal-free electrocatalysts were synthesized by ball-milling at high speed rotation (500 rpm) using stainless steel balls, generating sufficient kinetic energy to cause bond cleavages of the graphitic C-C framework. As a result, active carbon species formed at the broken edges of graphite, which were sufficiently reactive to pick up halogens in the sealed ball-mill capsule.
The resultant XGnPs were tested as cathode electrodes of fuel cells and revealed remarkable electrocatalytic activities for ORR with higher tolerance to methanol crossover/CO poisoning effects and longer-term stability than those of the original graphite and commercial Pt/C electrocatalysts.
Jong-Beom Baek, professor and director of the Interdisciplinary School of Green Energy/Low-Dimensional Carbon Materials Center, UNIST, Ulsan, South Korea, led the effort.
“Our result presents new insights and practical methods for designing edge-functionalized GnPs as high-performance metal-free ORR electrocatalysts through low-cost and scalable ball-milling techniques,” said Prof. Baek.
Jeon, I., Choi, H., Choi, M., Seo, J., Jung, S., Kim, M., Zhang, S., Zhang, L., Xia, Z., Dai, L., Park, N., & Baek, J. (2013). Facile, scalable synthesis of edge-halogenated graphene nanoplatelets as efficient metal-free eletrocatalysts for oxygen reduction reaction Scientific Reports, 3 DOI: 10.1038/srep01810
