To mix things up a bit, I've traded posts with Brandon from Chemtips, who's going to be telling you a bit more about Hanessian's pactamycin work in this special guest post. If you head over to his blog you can read my advice to anyone attempting a Birch reduction. Anyone else interested in guest blogging here or swapping posts, please contact me! Enjoy! -BRSM
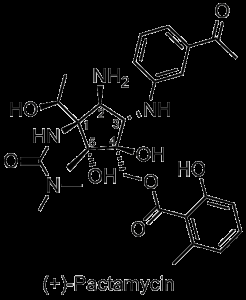
Today’s target, pactamycin, is an excellent example of just how creative evolution can be. Densely functionalized and almost unique (the authors put forward several loosely related structures), the Streptomyces metabolite is characterized by three contiguous tetrahedral carbons. First isolated in the early 1960’s, pactamycin binds strongly to the 30S fragment of the ribosome and is toxic to both mammalian and bacterial cells [1]. Briefly considered a potential anticancer agent, the natural product was ultimately shelved over safety concerns.
The first total synthesis of pactamycin was published by Hanessian et al. in Angew. Chem. Int. Ed. Eng. back in 2011, but I’m going to base most of this post on a follow-up publication in J. Org. Chem., which details some of the trials and tribulations encountered during the long synthesis [2]. The ACIE paper has also been discussed over at Synthetic Nature.
The synthesis starts off in the shallow end of the chiral pool, sequentially protecting L-threonine before thionyl chloride triggers cyclization into an oxazoline ring.
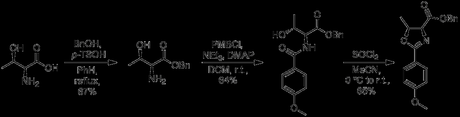
With all three functionalities thus hidden, a two-step aldol/silylation sequence links the oxazoline to an acrolein moiety, with the resulting stereochemistry dictated by the more stable Zimmerman-Traxler transition state. A spot of DIBAL-H reduces the benzyl ester down to an aldehyde, which then reacts with MeMgBr to give a secondary alcohol, subsequently oxidized back up to the ketone [3].
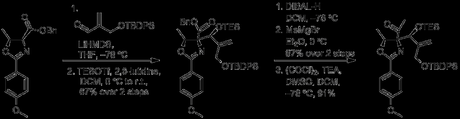
The authors then cleave the alkene, via treatment with ozone, to form a second ketone. This sets the stage for a second, TiCl4 mediated aldol, which forms the cyclopentane ring of pactamycin. Unfortunately, the tertiary alcohol produced during the aldol was a little more stable than they had hoped. Acetylation with Cl3CCOCl forced elimination to the alkene.
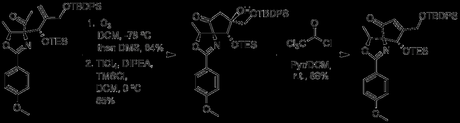
Its purpose complete, the ketone at C2 was then reduced to an alcohol (third redox at this site). The next step was to convert this alcohol into an azide, but all attempts to form a good leaving group led to decomposition. As a stop-gap, the authors thus protected the alcohol with acetic anhydride, and moved on to the C5 position.
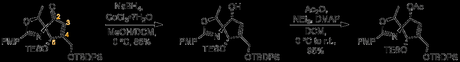
Acidic conditions cleaved the TES group while leaving the TBDPS unharmed, and the alcohol was oxidized to a ketone with DMP. Further attempts to form an azide at C2 also led to decomposition, and so the new ketone was converted to a tertiary center with MeMgBr. This new structure was no more amenable to formation of triflyl or mesyl esters, and so the synthesis lurched forward with functionalization of C3 and C4.
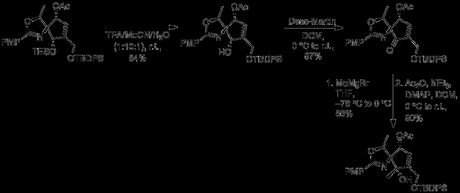
Removal of the TBDPS protecting group and treatment with m-CPBA led to epoxide formation. After a brief attempt at preparing a triflate ester at C2, the authors moved towards opening the epoxide ring with an aniline; however, early attempts led only to decomposition.
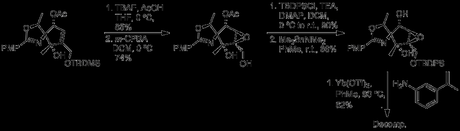
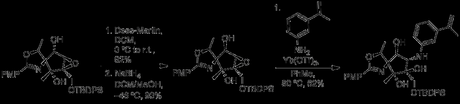
Not feeling optimistic about placing an azide at C2 in this new structure (and reluctant to add two steps to the synthesis just to revert the C2 stereochemistry), the authors decided to alter their order of transformations, moving back in the synthesis to the initial ring formation. Epoxidation while C2 was still a ketone proceeded smoothly with H2O2, and subsequently allowed for the crucial triflylation/substitution reaction. However, in the absence of a directing force the wrong epoxide stereochemistry was obtained.
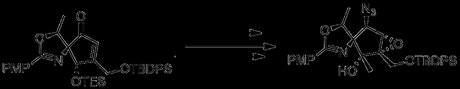
Placing the epoxidation reaction after reduction and protection of C2 allowed epoxidation with mCPBA, leading to the correct epoxide stereochemistry. Unfortunately, attempts to form the azide with this intermediate failed, and repeating the Grignard reaction detailed above led to the wrong stereochemistry at C5. Deciding that inverted epoxide of their previous attempt was the lesser evil, the authors returned to their second synthetic stream.
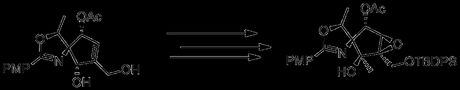
In a nice sequence of events, Zn(OTf)2 in hot acetic acid caused the epoxide to migrate to the primary alcohol, with the solvent then opening the ring. The acetate groups could then be removed with potassium carbonate in methanol, to give the requisite C4 stereochemistry in 87% yield.
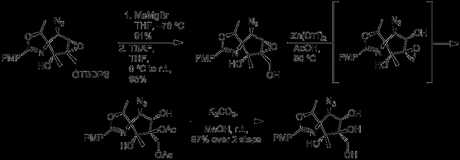
Re-protection of the primary alcohol with TBDPS-Cl and formation of a triflate ester at C3 regenerated the epoxide, this time with the necessary stereochemistry. The previous conditions with Yb(OTf)3 arylamine then opened the ring, completing the core of pactamycin. At this point in the paper the authors begin a lengthy excursion to synthesize a related analogue, pactamate, which in the interest of time I won’t get into. It does make for good reading though, and I recommend checking out the JOC paper.
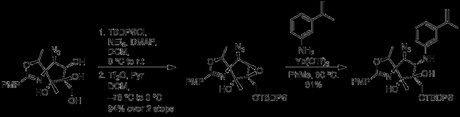
Following deprotection of the oxazoline, the last significant hurdle was formation of a dimethylurea at C1. Surprisingly, the free amine at this site had remarkably poor reactivity, to the extent that treatment with BnBr under relatively standard conditions (BnBr, NAH, TBAI in THF at 0℃) led to migration of the TBDPS group and formation of a primary benzyl ether, with the amine unharmed. As a testament to the difficulty of the scaffold, the authors had to rely on X-ray crystallography to assign this transformation.
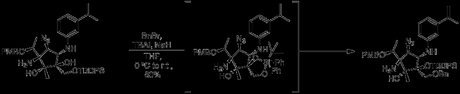
After at least five separate attempts at forming the urea, the group elected to just cover everything but the amine in protecting groups, allowing forcing conditions. The TBDPS group was thus converted into a hemiacetal with the nearby secondary alcohol, and the needed urea was formed via diphosgene and dimethylamine.
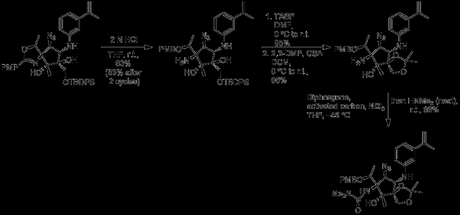
The last few steps of the synthesis are relatively routine, and simply remove the protecting groups and add an ester to the primary alcohol.
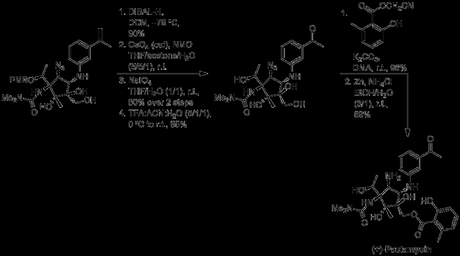
Breakdown of the synthesis.
33 linear steps
3% overall yield (reported by authors)
4 steps solely to protect FGs (triflates and such excluded)
10 redox reactions
It’s quite apparent from the convoluted sequence of reactions that this project was a beast to work on. Congratulations to Hanessian and colleagues for sticking with it, and for putting the project (warts and all) out for all to see.
[1] Or, at least that’s what Hanessian’s group reports. The reference for this info isn’t on Pubmed or google, which may stem from the fact it was published in the 1962 issue of Antimicrobial Agents and Chemotherapy, a journal that was founded in 1972. This may be evidence of time travel (ref 1), but more likely is due to an older journal with the same name that has never been put online.
[2] While researching for this post I discovered that the chemist who did most (all?) of this chemistry, Ramkrishna Reddy Vakiti, left Montreal recently. He’s currently working in Winnipeg, roughly a kilometer from my lab. To my knowledge we’ve never met.
[3] Convoluted redox reactions are common in this synthesis. Baran would not be pleased.
