 By weird coincidence, Salvador Herrando-Pérez (student blogger extra-ordinaire – see his previous posts on evolution, pollination, bird losses, taxonomic inflation, niche conservatism, historical biogeography, ecological traps and ocean giants) has produced a post this week expanding on the problem of roads. Also weirdly coincidental is that both Salva and I are in his home country of Spain this week.
By weird coincidence, Salvador Herrando-Pérez (student blogger extra-ordinaire – see his previous posts on evolution, pollination, bird losses, taxonomic inflation, niche conservatism, historical biogeography, ecological traps and ocean giants) has produced a post this week expanding on the problem of roads. Also weirdly coincidental is that both Salva and I are in his home country of Spain this week.
–
Australia’s > 800,000-km road network would go 60 times around the equator of our planet. Confined to the boundaries of any one country, roads are a conspicuous component of the landscape, and shape the dispersion, survival and reproduction of many plants and animals in urban and remote areas.
Those who drive (or are driven by) will be familiar with the image of a crushed kangaroo on the roadside (a hedgehog in Europe), or the sticky mosaic of insects smashed against the windscreen after a high-speed run. Mortality by collision is one of the many effects that roads can have on the demography of organisms – including humans. Those effects encompass
- physical alteration of terrestrial and aquatic habitats,
- chemical pollution leakage during road construction and maintenance, and from asphalt compounds during storms,
- alteration of animal behaviour (e.g., change in home range, or in patterns of flight or vocalisation),
- access to remote areas by hunters, fishermen and gatherers in general, and
- intense habitat fragmentation1-3.
However, some species get around those negative impacts by using the roads as pathways to new territories, thereby eluding barriers like seas, mountains, rivers, dense vegetation, or competition for vital resources with other species.
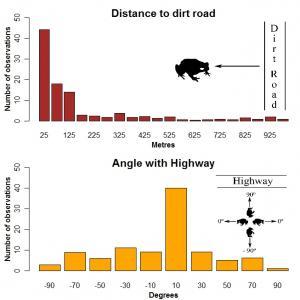
Figure 1: Road use by cane toads in the Northern Territory, Australia (2). Top panel: frequency of radio-tracked individuals recorded at different distances to the nearest dirt road (25 to 975 m in 50 m intervals). Bottom panel: frequency of toads with body axis aligned at different angles with the main axis of the Arnhem Highway (0º for toads facing the main axis of the road, to |90|º for toads facing perpendicular to the right or left of the road). Most toads were found within 25 m of dirt roads, and facing -10º to 10º to the highway axis.
Brown and colleagus4 illustrate the latter scenario with cane toads (formerly Bufo marinus; now Rhinella marina) in Australia. This species was introduced to Queensland in the 1930s to control (unsuccessfully) insect pests in sugar cane fields5. Nowadays, Aussie cane toads outnumber the human population in China, occupy a surface equivalent to 100 million soccer stadia, and only in 2009 crossed the border of Western Australia, > 2,500 km from the release point6 [see Rick Shine’s cane toad lab research]. For over 300 nights, Brown et al. radio-tracked 49 adult toads in farmland and eucalyptus forest in the Northern Territory. They found that these animals adjusted their movements to the local network of roads. In half of the records, toads were seen on or near dirt roads, mainly following those portions of the roads in the Northwest direction of progression of the invading front (Figure 1).
With an independent sample, they observed that the body axis of most individuals found near a highway was aligned with the principal axis of the road (Figure 1), giving evidence that the animals were moving relative to the trajectory of the highway. Undoubtedly, roads confer demographic benefits that compensate the > 200 tonnes of toads killed on the roads in (only) Queensland every year.
The invader’s backpack
Among the panoply of exotic species that are detrimental globally outside their native ranges7, the fungus Phytophthora lateralis is another self-explanatory example of road-loving biological invasion8. Rivers disperse the spores of this fungus. The spores then parasitise the roots of tree species along riverbanks and beyond (i.e., during floods), resulting in fulminating rot of the entire root system. In Oregon and California, spores have spread in most rivers in the mud stuck to vehicles and people’s shoes walking along local dirt roads. The disease was first recorded in the 1920s in Seattle in a plantation of Port Orford cedars (Chamaecyparis lawsoniana). It has since caused massive mortality of juvenile to centenary cedars in the conifer forests of Western USA, and has ruined a multi-million-dollar export of wood and ornamental trees (by means of which the fungus has already reached, at least, Asia and Europe).

Life-history highlights:
- One of the largest known anurans (> 20 cm, > 2.5 kg)
- Nocturnal as most amphibians
- Eurythermal: adults can tolerate from 10º to 43ºC
- They can lose > 50 % of body water in dry environments
- Main diet includes arthropods and carrion
- Females spawn from 6,000 up to ~35,000 eggs once or twice annually, preferably in shallow ponds
- Sexual maturity in 6-18 months
- Life span ~ 5 years
Roads also contribute to the spread of insect and plant pests and human diseases1,3. In the case of cane toads, dirt roads in the Northern Territory might be assisting in the dispersal of their toxicity (arguably the toad’s worst ecological impact6), present in all developmental stages (egg, tadpole, toadlet, and adult), especially for adults because toxicity increases with size. Up to 27 species of vertebrates have been reported to die from ingestion of cane toads5 – thus, at a population level, it is predictable that the worst ravages will occur in apex predators able to catch larger (and more toxic) toads, such as elapid snakes, varanid lizards, the freshwater crocodile (Crocodylus johnstoni) and quolls (Dasyurus spp.)6.
Surely we all want roads that are safe and (as much as possible) benign for biodiversity. Taylor and Goldingay2 have recently reviewed the state of the art of published research into roads and wildlife worldwide, with the following highlights:
- studies on large ungulates and carnivores predominate, partly due to rocketing insurance and medical costs after collisions;
- road-crossing structures for wild animals are being widely applied, although population benefits remain poorly understood;
- behavioural avoidance with genetic and metapopulation implications occurs in some species, calling for landscape road planning;
- focused management actions are needed for globally threatened species like amphibians (surely not cane toads),
- poor experimental designs across the board.
It caught my eye that the three reviews I cite1-3 did not encompass road impacts on subterranean ecosystems9 – a dark oasis of living wonders10 and reservoir of > 30 % (98 % along with glaciers) of available freshwater globally11. Clearly, to those who build roads (from the politician to the engineer), environmental impact assessment should take into account their multiple ecological impacts, and not only along the route itself2,3.
Next time you hit the road, be aware; you are never alone.
—
- S. C. Trombulak and C. A. Frissell, Conserv Biol 14 (1), 18 (2000) doi:10.1046/j.1523-1739.2000.99084.x
- B. D. Taylor and R. L. Goldingay, Wildl Res 37 (4), 320 (2010) doi:10.1071/WR09171
- W. F. Laurance, M. Goosem, and S. G. W. Laurance, Trends Ecol Evol 24 (12), 659 (2009) doi:10.1016/j.tree.2009.06.009
- G. P. Brown, B. L. Phillips, J. K. Webb et al., Biol Conserv 133 (1), 88 (2006) doi:10.1016/j.biocon.2006.05.020
- C. Lever, The Cane Toad. The History and Ecology of a Successful Colonist. (Westbury Academic and Scientific Publishing, Otley, UK, 2001)
- R. Shine, Quart Rev Biol 85 (3), 253 (2010) doi:10.1086/655116
- S. Lowe, M. Browne, and S. Boudjelas, Aliens 12, S1 (2000)
- E. M. Hansen, Boreal Environ Res 13, 33 (2008); E. M. Hansen, D. J. Goheen, E. S. Jules et al., Plant Disease 84 (1), 4 (2000) doi:10.1094/PDIS.2000.84.1.4; E. S. Jules, M. J. Kauffman, W. D. Ritts et al., Ecology 83 (11), 3167 (2002)
- M. Knez and T. Slabe, in Encyclopedia of Caves and Karst Science, edited by J. Gunn (Palgrave Macmillan, London, UK, 2004), pp. 419
- J. Gibert and L. Deharveng, BioScience 52 (6), 473 (2002) doi:10.1641/0006-3568(2002)052[0473:SEATFB]2.0.CO;2; D. C. Culver and B. Sket, J Cave Karst Stud 62 (1), 11 (2000)
- D. L. Danielopol, C. Griebler, A. Gunatilaka et al., Environmental Conservation 30 (2), 104 (2003) doi:10.1017/S0376892903000109
- B. L. Phillips, G. P. Brown, J. K. Webb et al., Nature 439 (7078), 803 (2006) doi:10.1038/439803a
- N. Sallam, Aust J Entomol 50 (3), 300 (2011) doi:10.1111/j.1440-6055.2010.00807.x

