
The Philippine bare-backed fruit bat (Dobsonia chapmani; body size = < 220 mm, < 150 g; IUCN status: ‘Critically Endangered A2cd’) is endemic to lowland rain forests [top habitat image] from Negros and Cebu islands. This species of flying fox had been missing from the 1970s and was declared extinct in 2002 (34). In May 2003, five specimens [one shown in the picture below] were trapped in night nets in the Calatong forest (Negros Island), a ~ 1,000-ha fragment of secondary rain forest and agricultural lands [bottom habitat image] (35). The species is reliant on fruit-bearing vegetation and caves for feeding and roosting, respectively. As with many other Philippine bats, it suffers from habitat degradation and hunting. The family Pteropodidae comprises > 150 species. Despite their Draculian look, they all feed on fruits and nectar, and act as important plant pollinators (36), as well as disease vectors such as Ebola virus (37). Flying foxes are distributed in the tropics and subtropics from the Eastern Mediterranean, through the Arabian Peninsula, Asia, Australia, and many islands of the Indian Ocean. Photos courtesy of Ely L. Alcala.
Jared Diamond (1) coined the expression ‘evil quartet’ for the four main human causes of species extinctions: habitat loss/fragmentation, overkill, introduced species and extinction chains [with climate change and extinction synergies (2), the updated expression would be ‘evil sextet”]. However, one third of ‘extinct’ mammal species has been ‘found’ again. Recent studies reveal that the probability of rediscovery depends on the cause of extinction.
Arriving in a city to search for an old friend, I would first look in the suburb where he lived, the pub where we enjoyed a drink and some music, or the park where we used to play football. But if my friend was an outlaw, or had recently gone through a traumatic experience, my chances of finding him at his favourite spots would shrink.
If, instead of a friend, we are searching for the last survivors of an extinct-declared species, surveys also tend to take place in the habitat in which the species was previously found. Such a strategy rests on the classical hypothesis that, given the spatial distribution of a species, its gradual decline must occur from the periphery to the core of its distribution (‘range collapse’) where, in theory, the habitat should be of better quality and the number of individuals higher (3). In contrast, recent work supports that the trajectory of demise of threatened vertebrates progresses from the core to the periphery (‘range eclipse’) (4), because many perturbations make their way as a progressive wave, e.g, fire, logging or urbanisation.
Diana Fisher (5) supports the ‘range eclipse’ hypothesis for ‘extinct’ mammals which have been rediscovered. She quantifies that 60% of the new records are made from peripheral habitats, mainly when the principal cause of extirpation is habitat loss. Not only that, on average species are rediscovered at altitudes 35 % higher than historical records, and only in 5 % of the cases at the locality where it had been last seen.
For instance, the thin-spined porcupine (Chaetomys subspinosus) was relocated in restinga and cocoa agroforestry plantations in Brazil (native habitat = Atlantic forest) (6), and the malabar civet (Viverra civettina) was rediscovered in thickets from degraded lowland vegetation and cashew plantations in India (native habitat = riparian vegetation) (7). Such findings emphasise that protection of peripheral habitats is worth considering if they act as reservoirs for threatened species. On the other hand, Fisher (5, 8) also predicted that rediscovery rates (hence, the most common mistake in reporting extinctions) will be highest for species extirpated by habitat loss but had formerly occupied large ranges. In contrast, species reduced by overexploitation (hunting, fishing, harvest) or introduction of foreign species, particularly when they had restricted distributions, are the best candidates for ‘real’ extinction.
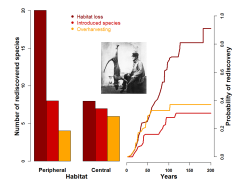
Rediscovery of ‘extinct’ mammals following extirpation by three human impacts. Bars indicate number of rediscoveries in the outer 50 % (periphery) and inner 50 % (core) of the known distribution areas (67 species) (5). Lines represent rediscovery probabilities from 0 to 200 years after species had been catalogued ‘extinct’ (187 species) (8). Rediscoveries are likelier (lines), and mostly in peripheral habitat (bars), when demographic collapse originates from habitat loss rather than overharvesting or introduction of foreign species. Overlaid, a hunter next to a Tasmanian tiger, a species which has not been seen in the last 80 years. In the first third of the 20th Century, the government of Tasmania provided a bounty for each adult and pup culled in an attempt to reduce sheep kills credited to this marsupial predator, and those hunts surely precipitated the extinction of the species.
Thus, > 20 surveys have failed to record the Tasmanian tiger (Thylacinus cynocephalus) in Australia (9) or the wild horse (Equus ferus) in Eurasia (10), both species being lost (mainly) to overkill (8). Likewise, feral cats and/or rats are attributed the extinction of endemic rodents on many islands worldwide, e.g., the mohuy (Brotomys voratus) in Haiti and Dominican Republic (11), or the Angel Island mouse (Peromyscus guardia) on the Mexican island of Ángel de la Guarda from Baja California (12).
To rediscover or not, does it matter?
The rediscovery of a thought-to-be-lost species become sensational news in the media, which can convey a wrong message (13): ‘this extinction business is not as bad as scientists claim’, and calls into question the work conservationists do. Thus, the (presumably) new record of the ivory-billed woodpecker (Campephilus principalis) in Arkansas (USA), on the basis on one observation (a 4-second video!) deserved a trail of papers in Science (14-17) and Auk (18-21), with arguments permeating through to ornithologists, managers and politicians:
“…it appears from the ivory-billed woodpecker events of the past year that public opinion is molded more by sound bites than by science. As seems inevitable, given the news media’s need for spontaneity and reporters covering subjects they know little about, bizarre and often misleading news articles have been widespread in both the general and birding-specific popular media” (19).
Most of the time however, rediscoveries are bound to “…documenting the prolonged demise of yet another species” (13). So > 85 % of the ~ 350 species of amphibians, birds and mammals, considered to be extinct but then rediscovered, have an IUCN status from ‘vulnerable’ to ‘critically endangered’ (22). Only the rediscovery of a viable population matters to conservation. Our estimates of extinction rates are subject to uncertainty (23), as illustrated by recent debates about their estimation through species-area curves (24-27), or for some of the best-studied biota worldwide (28-30). These caveats ultimately originate from our inability and/or lack of resources to monitor entirely the potential habitat of most species on the planet. They are further exacerbated because biological surveys concentrate on large, charismatic taxa often (31), and in temperate regions (32), and also because many rediscoveries go unnoticed in grey literature or local journals.
It is puzzling to realise that many of the taxa we know have only been seen once (i.e., when they were originally described); so, are they extinct? (32, 33). All in all, the complex connotations (emotional, economical, political, scientific) of the concept ‘extinction’ underline a challenge we ecologists face in our modern communication with society – to inform clearly and objectively about the uncertainties inherent to assigning conservation status to the species we study.
Literature cited
- J. M. Diamond, in Conservation for the twenty-first century, D. Western, M. C. Pearl, Eds. ( Oxford University Press, London, UK, 1989), pp. 37-41
- B. W. Brook, N. S. Sodhi, C. J. A. Bradshaw, Trends Ecol. Evol. 23, 453 (Aug, 2008)
- R. D. Sagarin, S. D. Gaines, Ecol. Lett. 5, 137 (Jan, 2002)
- R. Channell, M. V. Lomolino, Nature 403, 84 (Jan, 2000)
- D. O. Fisher, Glob. Ecol. Biogeogr. 20, 415 (May, 2011)
- I. B. Santos, W. L. R. Oliver, A. B. Rylands, Dodo 24, 43 (1987)
- G. U. Kurup, Tigerpaper (Bangkok) 16, 13 (1989)
- D. O. Fisher, S. P. Blomberg, Proc. R. Soc. B. 278, 1090 (Apr, 2011)
- N. Mooney, D. E. Rounsevell, in The Mammals of Australia, S. Van Dyck, R. Strahan, Eds. (Reed New Holland, Sydney, Australia, 2008), pp. 167-168
- O. A. Ryder, Putting the wild horse back into the wild. Przewalski’s Horse Global Conservation Plan. (Zoological Society of San Diego, Center for Reproduction of Endangered Species, San Diego, CA, USA, 1990)
- C. A. Woods, C. W. Kilpatrick, in Mammal species of the world: A taxonomic and geographic reference, D. E. Wilson, D. M. Reeder, Eds. (Johns Hopkins University Press, Baltimore, USA, 2005), pp. 1538-1600
- S. T. Alvarez-Castañeda, A. Ortega-Rubio, Biol. Conserv. 109, 157 (Feb, 2003)
- R. J. Ladle, P. Jepson, S. Jennings, A. C. M. Malhado, Nature 461, 723 (Oct, 2009)
- J. W. Fitzpatrick et al., Science 308, 1460 (Jun, 2005)
- J. W. Fitzpatrick, M. Lammertink, M. D. Luneau, T. W. Gallagher, K. V. Rosenberg, Science 311, 1555b (Mar, 2006)
- D. A. Sibley, L. R. Bevier, M. A. Patten, C. S. Elphick, Science 311, (Mar, 2006)
- D. A. Sibley, L. R. Bevier, M. A. Patten, C. S. Elphick, Science 315, 1495 (Mar, 2007)
- J. A. Jackson, Auk 123, 1185 (Oct, 2006)
- J. A. Jackson, Auk 123, 1 (Jan, 2006)
- J. W. Fitzpatrick et al., Auk 123, 587 (2006)
- J. W. Fitzpatrick et al., Auk 123, 1189 (Oct, 2006)
- B. R. Scheffers, D. L. Yong, J. B. C. Harris, X. L. Giam, N. S. Sodhi, PLoS One 6, (Jul, 2011)
- N. E. Stork, Biodivers. Conserv. 19, 357 (Feb, 2010)
- H. M. Pereira, L. Borda-de-Agua, I. S. Martins, Nature 482, E3 (Feb, 2012)
- C. D. Thomas, M. Williamson, Nature 482, E4 (Feb, 2012)
- F. L. He, S. P. Hubbell, Nature 482, E5 (Feb, 2012)
- F. L. He, S. P. Hubbell, Nature 473, 368 (May, 2011)
- J. A. Thomas, R. T. Clarke, Science 305, 1563 (Sep, 2004)
- C. Hambler, M. R. Speight, Science 305, 1563 (Sep, 2004)
- J. A. Thomas et al., Science 303, 1879 (Mar, 2004)
- D. O. Fisher, Biol. Conserv. 144, 1712 (May, 2011)
- J. M. Diamond, Conserv. Biol. 1, 77 (1987)
- F. W. King, Conserv. Biol. 2, 395 (Dec, 1988)
- L. R. Heaney, E. K. Walker, B. R. Tabaranza, N. R. Ingle, Sylvatrop 10, 6 (2002)
- E. L. Alcala, R. B. Paalan, L. T. Averia, A. C. Alcala, Siliman J, 45, 123 (2004)
- A. G. Marshall, Biol. J. Linnean Soc. 20, 115 (1983)
- E. M. Leroy et al., Nature 438, 575 (2005)
-34.917731 138.603034

