About fenbendazole
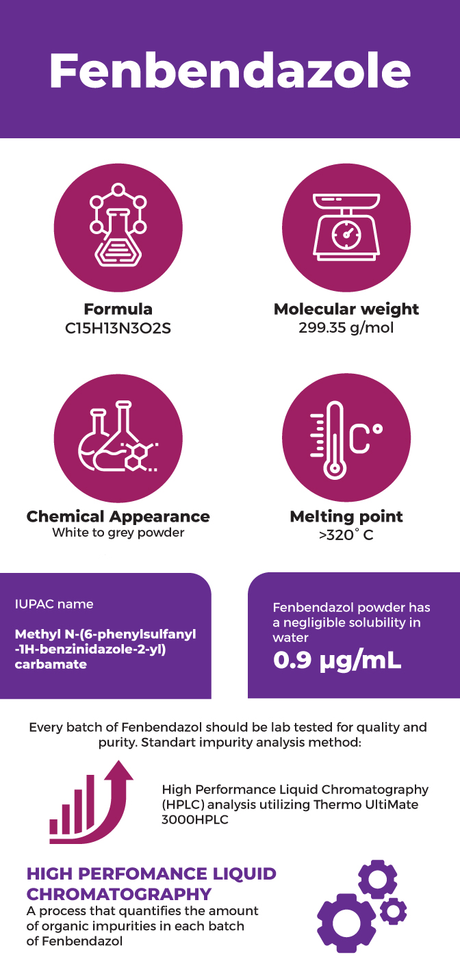
Fenbendazole IUPAC name – methyl N-(6-phenylsulfanyl-1H-benzinidazole-2-yl) carbamate
Formula: C15H13N3O2S
Molecular weight: 299.35 g/mol
Chemical Appearance – White to gray powder
Fenbendazol powder has a negligible solubility in water – 0.9 µg/mL.
Melting point of >320° C.
Every batch of Fenbendazole is lab tested for quality and purity. Standart impurity analysis method: High Performance Liquid Chromatography (HPLC) analysis utilizing Thermo UltiMate 3000HPLC High Perfomance Liquid Chromatography – a process that quantifies the amount of organic impurities in each batch of Fenbendazol. The test results are used to measure the purity level of the finished product. Every batch of Fenbendazol is tested this way to ensure the maximum success rate for product quality and effectiveness. The certificate of analysis confirms that the Fenbendazole powder is one of the most reliable products of this category. Our Analysis Report goes into greater detail to describe the merits of Fenbendazole.
It is necessary to mention that for the quality assurance, finished products are tested at the end of the production process – while only a small number of samples are tested at any stage of processing.
Learn More!