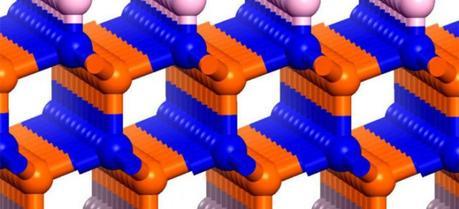
 Illustration of the boron-nitride bilayer (blue and orange atoms) functionalized with fluorine atoms along the bottom and hydrogen atoms long the top. (Credit: Jinlong Yang / University of Science and Technology of China)
Illustration of the boron-nitride bilayer (blue and orange atoms) functionalized with fluorine atoms along the bottom and hydrogen atoms long the top. (Credit: Jinlong Yang / University of Science and Technology of China)
Infrared light could help split water into hydrogen and oxygen, despite the fact that infrared photons have less energy than is needed to drive the reaction. That is the claim of physicists in China, who have calculated that the reaction could proceed with the help of a bilayer catalyst that has a strong internal electric dipole.
While making this catalyst in the lab would be very difficult, the researchers are now trying to come up with a more practical alternative. If they are successful, such catalysts would allow a far larger proportion of the solar spectrum to be used to generate hydrogen—perhaps making it a commercially viable source of hydrogen fuel.
Photochemical splitting is usually realized in a suspension of catalyst particles in water. A particle absorbs a solar photon, which generates an electron–hole pair that stimulates the decomposition of water and liberates the hydrogen. In order to succeed, the band-gap energy associated with the creation of the electron–hole pair must be greater than 1.23 eV—which is the minimum needed to free-up the hydrogen. This corresponds to a near-infrared photon, so in theory photons with at least this energy (about 57% of photons from the Sun) can split water molecules.
Now, Jinlong Yang and colleagues at the University of Science and Technology of China in Hefei have set their sights on an even more ambitious goal: to make use of much more of the solar spectrum by developing a catalyst that works with infrared light well below the 1.23 eV threshold. While this might sound impossible from an energy point of view, catalysts often work by dividing a chemical process into several steps, each of which requires less energy than the overall process.
Using advanced computational algorithms based on density functional theory, the team designed an ultrathin catalyst comprising a bilayer of boron nitride functionalized with hydrogen atoms on the upper surface and fluorine atoms on the lower one. Fluorine has a very large electronegativity, which means that valence electrons would migrate to the lower surface of the bilayer to cause a potential difference of about 10 V between the top and bottom of the device.
The team calculates that the material has a band gap of just 0.85 eV, thereby allowing it to absorb infrared photons. The research suggests that when an electron–hole pair is created, the electron migrates to the top surface and the hole to the bottom surface, with the electron–hole pair thereby gaining 10 eV of energy. This is more than enough energy to split water and the researchers predict that hydrogen from water molecules would be reduced to hydrogen gas by the free electrons at the top surface, whereas oxygen from other water molecules would be oxidized to oxygen gas by the holes at the bottom.
There is a catch, however. The process leaves negative hydroxide ions bound to the top surface and positive hydrogen ions attached to the bottom. These ions would reduce the electric field across the catalyst, and photodecomposition would soon stop. The researchers suggest a pulsed electric field could remove the ions, but this would require additional energy and so would therefore reduce the efficiency of the process. However, any reduction in efficiency would be outweighed by the catalyst’s ability to exploit the energy of infrared photons.
Creating the exact catalyst proposed by the team would be a challenging and costly process. First it would involve making two boron-nitride monolayers. Then each monolayer would need to be functionalized separately and finally joined together back-to-back. Yang and colleagues are now working with experimentalists to try to develop alternative structures in the lab that yield the same results. “We are trying to find new materials that could be synthesized in bulk and then reduced down to the nanoscale,” says Yang. “These would then be easy to produce industrially.”
Full article by Tim Wogan is available on the Physics World website.
Xingxing Li, Zhenyu Li, Jinlong Yang (2014). Proposed Photosynthesis Method for Producing Hydrogen from Dissociated Water Molecules Using Incident Near-Infrared Light Physical Review Letters, 112 (1) DOI: 10.1103/PhysRevLett.112.018301
