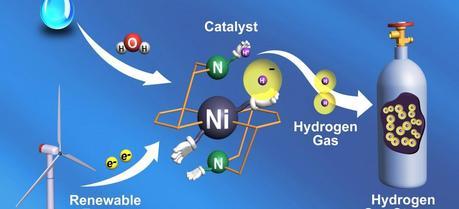
 (Courtesy of Pacific Northwest National Laboratory)
(Courtesy of Pacific Northwest National Laboratory)Chemists at the Ruhr-University Bochum, Germany, have developed a carbon-based catalyst for water electrolysis and hydrogen combustion.
When energy is supplied, the so-called bi-functional catalysts can split water into hydrogen and oxygen. They can then store the energy in the chemical bonds of the thus formed hydrogen. The same catalysts can also have their polarity reversed to become fuel cells; they combust hydrogen with oxygen to water, generating electricity at the same time. So far, researchers have been using noble-metal catalysts for this purpose. However, these catalysts have the disadvantage of being either good for electrolysis or good for combustion, but not for both.
SEE ALSO: Cheap, Abundant, Low-Toxic Photocatalyst Discovered
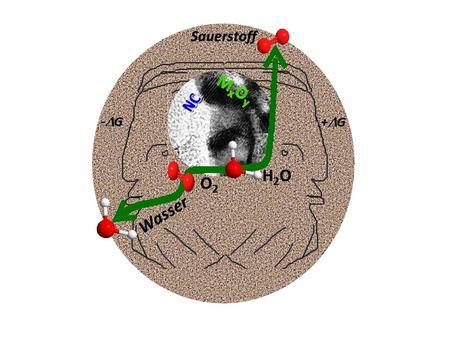
Janus-faced catalyst: The novel catalysts developed by RUB chemists facilitate two opposite reactions: the electrolysis of water and the combustion of hydrogen with oxygen. (Credit: Ruhr-University Bochum / Masa)
The novel catalysts from Bochum are made from manganese-oxide or cobalt-oxide nanoparticles which are embedded in specially modified carbon, into which the researchers have integrated nitrogen atoms in specific positions. The team headed by Prof Dr Wolfgang Schuhmann and Prof Dr Martin Muhler from the Faculty of Chemistry and Biochemistry analysed the catalysts using a number of spectroscopic and electrochemical methods. They have thus determined which properties are essential for bi-functionality.
In a previous publication in the Journal of the American Chemical Society, the researchers from Bochum described another approach for manufacturing bi-functional carbon catalysts. To this end, they had “cut open” carbon nanotubes by applying thermal energy and oxygen, thus rendering the catalyst particles embedded therein usable. “This method is irresistibly simple,” says Martin Muhler. Compared to noble-metal catalysts, the production would be highly cost-efficient.
The German Research Foundation backed the research as part of the Excellence Cluster RESOLV (EXC 1069), and the Helmholtz-Energie-Allianz as part of the project “Stationary electrochemical storage and conversion systems” (HA-E-0002).
Masa, J., Xia, W., Sinev, I., Zhao, A., Sun, Z., Grützke, S., Weide, P., Muhler, M., & Schuhmann, W. (2014). MnO/NC and CoO/NC Nanoparticles Embedded in a Nitrogen-Doped Carbon Matrix for High-Performance Bifunctional Oxyg Angewandte Chemie International Edition DOI: 10.1002/anie.201402710
