 Another corker from Salvador Herrando-Pérez:
Another corker from Salvador Herrando-Pérez:
–
Cinema fans know that choosing a movie by the newspaper’s commentary or the promotional poster might be a lottery. In the movie of nature, to confuse ‘the attractive’ with ‘the appropriate’ can compromise the life of an individual and its offspring, even to the extent of anticipating the extinction of an entire population or species.
Animals make daily choices about when, where or with whom to engage in basic activities like eating, hibernating, mating, migrating or resting. Those choices are often strongly tied to highly specific cues – e.g., air temperature, tree density, location of water, or smell of other individuals. And it happens to hair lice jumping from head to head among school kids, or to caribou forming their winter herds prior to the seasonal migration. All species, without exception, persist in nature because those ‘choices’ translate into survival or successful reproduction more often than do not. They are a kind of evolutionary memory imprinted in an organism’s genes and behaviour. However, sometimes the right choice (‘right’ meaning perceiving a cue for the role it actually has in the life cycle) places an individual in the worst of all possible situations. The environment cheats, ‘the attractive’ merely mimics ‘the appropriate’, and the individual fails to reproduce, starves, sickens, or even dies.

Figure 1. Water reservoirs tainted with fuel (see dark contours) in Kuwait following the Gulf War in the early 1990s. Overlaid pictures show the silhouettes of trapped odonates (right), vertebrates (top left) and invertebrates (bottom left) (Photos courtesy of Jochen Zeil).
At the mercy of mirages
During the Gulf War, the destruction of infrastructure for crude exploitation spilled large amounts of fuel in many water reservoirs over the desert landscape of Kuwait. A little later, Horváth and Zeil1 found agglomerations of dead insects (and a range of vertebrates) along the shores of these polluted reservoirs, and observed dragonflies drowning in their kamikaze attempt to spawn on the oily surface (Figure 1). This work stimulated further research whereby Horváth and his team in Budapest showed that odonates are attracted by light polarization at the surface of oiled water2 – hence ‘polarized light pollution’3. Not only that, they recorded insects struggling to spawn on or mate with riveting surfaces such as solar panels, asphalted roads, plastic bags or (creepy enough!) cemetery crypts4. It goes without saying: these insects are victims of a mirage.
Those habitats or features of the habitat that mislead an animal’s choice, often hampering the completion of its life cycle, are known as ‘ecological traps’ – in other words, the environmental cue is decoupled from the quality of the habitat it is meant to signal. Ecological traps were first described in the 1970s by Dwernychuk and Boag5. They found that ducks on the islands of Miquelon lake located their nests among those of seagulls despite the latter happily devoured their ducklings and eggs. When these islands emerged in the middle of last century, they were first colonized by common terns (Sterna hirundo). By defending their own nests ferociously from predators (mainly crows and magpies), the terns inadvertently shielded the nests of their ducky comrades. The Canadians hypothesized that when seagulls subsequently replace terns, the ducks continued to sense their new neighbours as a (now misleading) sign of protection.
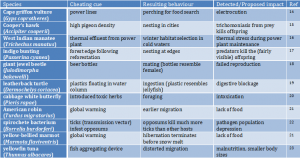
Table 1. Examples of hypothesised ecological traps (more in 6,13). This phenomenon warns that where we see animals might not necessarily be where they do their best.
More recently, ecological traps have been hypothesised in scenarios where the trapping cue is a sudden human-made change to which organisms have not adapted an evolutionary response, thus an ‘evolutionary trap’6. Those scenarios can be both dramatic and grotesque (Table 1) – for instance, males of Cuban treefrogs (Osteopilus septentrionalis) copulating with road-killed females (females stop moving and vocalising when receptive)7, or hatchlings of loggerhead sea turtles (Caretta caretta) venturing towards a fictitious sea reflected in the glossy walls and windows of tourist resorts8.
Attractive sinks
Conceptually speaking, ecological traps are ‘attractive sinks’ because they absorb immigrants but produce zero emigrants9. They can trigger extinction if all individuals of a population or species are driven into the trap, all the more likely an outcome for endangered populations10. Therefore, this phenomenon matters to conservation and management6,11, calls for a better understanding of the relationship between habitat quality and habitat selection in popular research areas such as metapopulation dynamics or species distribution modelling12, and sorely necessitates robust methods of measurement. Indeed, to confirm an ecological trap, it is necessary to quantify that organisms choose the trap with equal or larger probability than a genuinely good habitat, and that reproduction and/or survival (fitness) are reduced in the trap relative to other available habitats 13. Robertson and Hutto13 revised the thriving literature of ecological traps and found few studies that provide such combined evidence, reflecting that ecological traps might be rare, or authors might be failing to detect them.
When an ecological trap does occur and the cheating cue can be unequivocally identified, Gilroy and Sutherland11 suggest that we might be able to avoid population impacts by (i) creating strong settlement cues in alternative habitats (e.g., nesting in woodland core against predator-scrutinised edges by reducing clear-fells), (ii) improving the quality of the trap (e.g., mowing agricultural fields only after bird chicks have fledged to prevent nest failure), or (iii) eliminating or attenuating the cheating cue in the habitat trap (e.g., Horváth, and colleagues9 achieved this for insects tricked by adding nonpolarising white tape around the cell borders of solar panels). Option (iii) would be ideal for Kuwait water reservoirs – avoid war.
Literature
1 Horváth, G. & Zeil, J. Kuwait oil lakes as insect traps. Nature 379, 303-304, doi:10.1038/379303a0 (1996)
2 Horváth, G., Bernáth, B. & Molnár, G. Dragonflies find crude oil visually more attractive than water: multiple-choice experiments on dragonfly polarotaxis. Naturwissenschaften 85, 292-297 (1998)
3 Horváth, G., Kriska, G., Malik, P. & Robertson, B. Polarized light pollution: a new kind of ecological photopollution. Frontiers in Ecology and the Environment 7, 317-325, doi:10.1890/080129 (2009)
4 Horváth, G., Malik, P., Kriska, G. & Wildermuth, H. Ecological traps for dragonflies in a cemetery: the attraction of Sympetrum species (Odonata : Libellulidae) by horizontally polarizing black gravestones. Freshwater Biology 52, 1700-1709, doi:10.1111/j.1365-2427.2007.01798.x (2007)
5 Dwernychuk, L. W. & Boag, D. A. Ducks nesting in association with gulls – an ecological trap? Canadian Journal of Zoology 50, 559-563, doi:10.1139/z72-076 (1972)
6 Schlaepfer, M. A., Runge, M. C. & Sherman, P. W. Ecological and evolutionary traps. Trends in Ecology and Evolution 17, 474-480, doi:10.1016/S0169-5347(02)02580-6 (2002)
7 Meshaka, W. E. Anuran Davian behavior: a darwinian dilemma. Florida Science 59, 74-75 (1996)
8 Witherington, B. E. in Behavioral Approaches to Conservation in the Wild (eds. J. R. Clemmons & R. Bucholz) pp. 303-328 (Cambridge University Press, 1997)
9 Delibes, M., Ferreras, P. & Gaona, P. Attractive sinks, or how individual behavioural decisions determine source-sink dynamics. Ecology Letters 4, 401-403, doi:10.1046/j.1461-0248.2001.00254.x (2001)
10 Kokko, H. & Sutherland, W. J. Ecological traps in changing environments: ecological and evolutionary consequences of a behaviourally mediated Allee effect. Evolutionary Ecology Research 3, 537-551 (2001)
11 Gilroy, J. J. & Sutherland, W. J. Beyond ecological traps: perceptual errors and undervalued resources. Trends in Ecology and Evolution 22, 351-356, doi:10.1016/j.tree.2007.03.014 (2007)
12 Battin, J. When good animals love bad habitats: ecological traps and the conservation of animal populations. Conservation Biology 18, 1482-1491, doi:10.1111/j.1523-1739.2004.00417.x (2004)
13 Robertson, B. A. & Hutto, R. L. A framework for understanding ecological traps and an evaluation of existing evidence. Ecology 87, 1075-1085, doi:10.1890/0012-9658(2006)87[1075:AFFUET]2.0.CO;2 (2006)
14 Mundy, P. J. in Vulture Biology and Management (ed. S.R. Wilbur) pp. 57–74 (University of California Press, 1983)
15 Boal, C. W. & Mannan, R. W. Comparative breeding ecology of Cooper’s hawks in urban and exurban areas of Southeastern Arizona. Journal of Wildlife Management 63, 77-84, doi:10.2307/3802488 (1999)
16 Shane, S. H. Manatee use of power plant effluents in Brevard County, Florida. Florida Science 47, 180–187 (1984)
17 Weldon, A. J. & Haddad, N. M. The effects of patch shape on indigo buntings: evidence for an ecological trap. Ecology 86, 1422-1431, doi:10.1890/04-0913 (2005)
18 Gwynne, D. T. & Rentz, D. C. F. Beetles on the bottle: male buprestids mistake stubbies for females (Coleoptera). Australian Journal of Entomology 22, 79-80, doi:10.1111/j.1440-6055.1983.tb01846.x (1983)
19 Bjorndal, K. A., Bolten, A. B. & Lagueux, C. J. Ingestion of marine debris by juvenile sea turtles in coastal Florida habitats. Marine Pollution Bulletin 28, 154-158, doi:10.1016/0025-326x(94)90391-3 (1994)
20 Chew, F. Food plant preferences of Pieris caterpillars. Oecologia 46, 347-353 (1980)
21 Inouye, D. W., Barr, B., Armitage, K. B. & Inouye, B. D. Climate change is affecting altitudinal migrants and hibernating species. Proceedings of the National Academy of Sciences of the USA 97, 1630-1633, doi:10.1073/pnas.97.4.1630 (2000)
22 Keesing, F. et al. Hosts as ecological traps for the vector of Lyme disease. Proceedings of the Royal Society of London B 276, 3911-3919, doi:10.1098/rspb.2009.1159 (2009)
23 Hallier, J.-P. & Gaertner, D. Drifting fish aggregation devices could act as an ecological trap for tropical tuna species. Marine Ecology Progress Series 353, 255-264, doi:10.3354/meps07180 (2008)
-34.917731 138.603034
