Carnivores are essential components of trophic webs, and ecosystem functions crumble with their loss. Novel data show the connection between calcareous reefs and sea otters under climate change.
 Trophic cascade on the Aleutian Islands (Alaska, USA) linking sea otters (Enhydra lutris) with sea urchins (Strongylocentrotus polyacanthus) and calcareous reefs (Clathromorphum nereostratum). With males weighting up to 50 kg, sea otters have been IUCN-catalogued as Endangered since 2000. The top photo shows a male in a typical, belly-up floating position. The bottom photo shows live (pinkish) and dead (whitish) tissue on the reef surface as a result of grazing of sea urchins at a depth of 10 m. Sea otters are mesopredators, typically foraging on small prey like sea urchins, but their historical decline due to overhunting unleashed the proliferation of the echinoderms. At the same time, acidification and sea-water warming have softened the skeleton of the reefs, allowing for deeper grazing by sea urchins that eliminate the growth layer of living tissue that give the reefs their pinkish hue. Large extents of dead reefs stop fixing the excess in carbonic acid, whose carbon atoms sea water sequesters from the atmosphere enriched in carbon by our burning of fossil fuels. Photos courtesy of Joe Tomoleoni taken in Moss Landing – California, USA (otter), and on the Near Islands – Aleutian Archipelago, Alaska (reef).
Trophic cascade on the Aleutian Islands (Alaska, USA) linking sea otters (Enhydra lutris) with sea urchins (Strongylocentrotus polyacanthus) and calcareous reefs (Clathromorphum nereostratum). With males weighting up to 50 kg, sea otters have been IUCN-catalogued as Endangered since 2000. The top photo shows a male in a typical, belly-up floating position. The bottom photo shows live (pinkish) and dead (whitish) tissue on the reef surface as a result of grazing of sea urchins at a depth of 10 m. Sea otters are mesopredators, typically foraging on small prey like sea urchins, but their historical decline due to overhunting unleashed the proliferation of the echinoderms. At the same time, acidification and sea-water warming have softened the skeleton of the reefs, allowing for deeper grazing by sea urchins that eliminate the growth layer of living tissue that give the reefs their pinkish hue. Large extents of dead reefs stop fixing the excess in carbonic acid, whose carbon atoms sea water sequesters from the atmosphere enriched in carbon by our burning of fossil fuels. Photos courtesy of Joe Tomoleoni taken in Moss Landing – California, USA (otter), and on the Near Islands – Aleutian Archipelago, Alaska (reef).For most, the decisions made by people we have never met affect our daily lives. Other species experience the same phenomenon because they are linked to one another through a trophic cascade.
A trophic cascade occurs when a predator limits the abundance or behavior of its prey, in turn affecting the survival of a third species in lower trophic levels that have nothing directly to do with the predator in question (1).
Sea otters (Enhydra lutris) represent a text-book example of a trophic cascade. These mustelids (see video footage here and here) hunt and control the populations of sea urchins (Strongylocentrotus polyacanthus), hence favouring kelp forests — the fronds of which are eaten by the sea urchins.
Removing the predator from the equation should lead to more sea urchins and less kelp, and this chain of events is exactly what happened along the coasts of the North Pacific (2, 3). The historical distribution of sea otters once ranged from Japan to Baja California through the Aleutian Islands (see NASA’s photo from space, and documentary on the island of Unimak), a sub-Arctic, arc-shaped archipelago including > 300 islands between Alaska (USA) and the Kamchatka Peninsula (Russia), extending ~ 2000 kilometres, and having a land area of ~ 18,000 km2.
But the fur trade during the 18th and 19th centuries brought the species to the brink of extinction, down to < 2000 surviving individuals (4). Without otters, sea urchins boomed and deforested kelp ecosystems during the 20th Century (5). Now we also know that this trophic cascade has climate-related implications in other parts of the marine ecosystem.
Underwater bites
Doug Rasher and collaborators have studied the phenomenon on the Aleutian Islands (6). The seabed of this archipelago is a mix of sandy beds, kelp forests, and calcareous reefs made up of calcium and magnesium carbonates fixed by the red algae Clathromorphum nereostratum. These reefs have grown at a rate of 3 cm annually for centuries as the fine film of living tissue covering the reef takes the carbonates from the seawater (7).
Essentially, the reef thickens towards the bottom of the sea and its hardness prevents overgrazing by herbivores. Rasher and colleagues measured the depth of the scars inflicted by the jaws of the sea urchins on the surface of the reefs (bioerosion) and found that some islands have lost up to 80% of their growth layer. The loss of this layer renders the reef functionally dead.
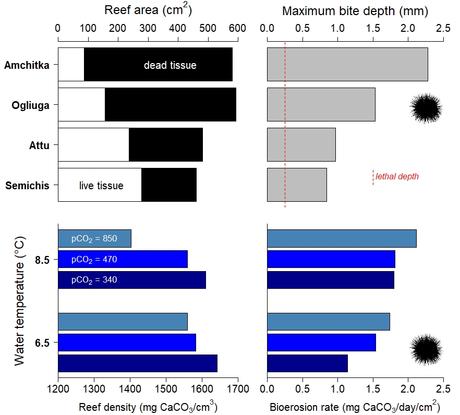
Parallel experiments in aquaria by the same team have shown that the density of the reef skeleton decreases in response to increased acidification and water temperature, thus mimicking the impacts that climate change has exerted on the Aleutian reefs in natural conditions (8).
From the Industrial Revolution, acidification and sea-water warming have softened the reefs. The proliferation of sea urchins resulting from sea-otter declines has magnified the magnitude of lethal bioerosion because the spiny-skinned invertebrates are now able to bite deeper into the reef.
Investing in otters
Calcareous reefs are true forms of submarine engineering, creating a splendid 3-D rocky seascape that combats coastal erosion and recruits myriad fish and invertebrates (9).
At larger scales, and like kelp forests (10), the reefs act as gigantic sinks of the excess carbon generated by the burning of fossil fuels (11). Because many molecules of calcium carbonate fixed by the reefs are molecules of carbonic acid that are removed from the seawater, maintaining the components of the otter-urchin-algae trophic cascade implies investing in methods to limit ocean acidification.
Fortunately, conservation actions and reintroduction of sea otters that have been happening since the 1970s have augmented their numbers; recent estimates place the overall population at some 130,000 individuals that currently occupy over half of their historical range (4).
The bad news is that global sea-otter populations are still declining. And, with < 9000 individuals, the Aleutian populations have not recovered from the fur trade, while now possibly facing overhunting by sharks and killer whales (12).
Be it on land or at sea, the reintroduction of predators in their native habitats will often conflict with economic sectors benefiting from their prey. In the case of sea otters, such conflicts emerge between otters and commercial fisheries of some invertebrates, such as abalone (13) and crabs (14).
However, the net value of the presence of sea otters is worth €32M per year for carbon sequestration, increased fish numbers, and ecotourism (15). These marine mammals are a clear instance of the public advantages derived from the reintroduction of native fauna compensate for the unavoidable losses that it might also entail (16).
Note: A Spanish version of this blog was published by the journal Quercus in January 2022.
References
- Silliman BR & Angelini, C (2012) Trophic cascades across diverse plant ecoystems. Nature Education Knowledge 3: 44
- Estes JA & Duggins, DO (1995) Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm. Ecological Monographs 65: 75-100
- Estes JA & Palmisano, JF (1974) Sea otters: their role in structuring nearshore communities. Science 185: 1058-1060
- Davis RW et al. (2019) Future directions in sea otter research and management. Frontiers in Marine Science 5: 510
- Steneck RS et al. (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation 29: 436-459
- Rasher DB et al. (2020) Keystone predators govern the pathway and pace of climate impacts in a subarctic marine ecosystem. Science 369: 1351-1354
- Frantz BR, Foster, MS & Riosmena-Rodríguez, R (2005) Clathromorphum nereostratum (Corallinales, Rhodophyta): the oldest alga? Journal of Phycology 41: 770-773
- Chan PTW et al. (2019) Recent density decline in wild-collected subarctic crustose coralline algae reveals climate change signature. Geology 48: 226-230
- Nelson WA (2009) Calcified macroalgae critical to coastal ecosystems and vulnerable to change: a review. Marine and Freshwater Research 60: 787-801
- Wilmers CC et al. (2012) Do trophic cascades affect the storage and flux of atmospheric carbon? An analysis of sea otters and kelp forests. Frontiers in Ecology and the Environment 10: 409-415
- van der Heijden LH & Kamenos, NA (2015) Reviews and syntheses: calculating the global contribution of coralline algae to total carbon burial. Biogeosciences 12: 6429-6441
- Tinker MT et al. (2021) Sea otter population collapse in southwest Alaska: assessing ecological covariates, consequences, and causal factors. Ecological Monographs 91: e01472
- Raimondi P, Jurgens, LJ & Tinker, MT (2015) Evaluating potential conservation conflicts between two listed species: sea otters and black abalone. Ecology 96: 3102-3108
- Boustany AM et al. (2021) Examining the potential conflict between sea otter recovery and dungeness crab fisheries in California. Biological Conservation 253: 108830
- Gregr EJ et al. (2020) Cascading social-ecological costs and benefits triggered by a recovering keystone predator. Science 368: 1243-1247
- Carswell LP, Speckman, SG & Gill, VA (2015) in Sea Otter Conservation, SE Larson, JL Bodkin & GR VanBlaricom, Eds. (Academic Press, Boston), pp. 333-368

