Total Synthesis of (–)-Nakadomarin A
David A. Evans et. al., J. Am. Chem. Soc., 2013, Just Accepted [PDF] [SI] [GROUP]
DOI: 10.1021/ja404673s
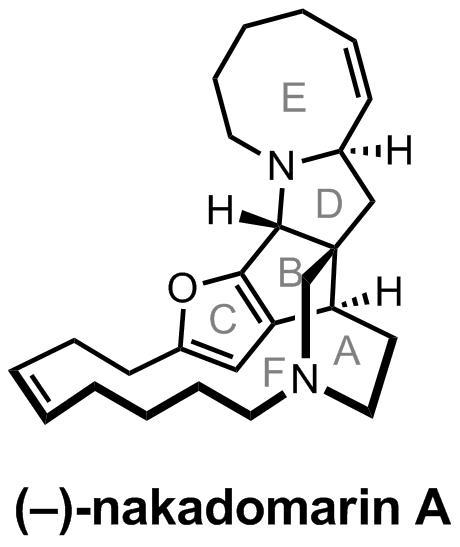
Of course, I’m not too surprised that people are still interested in making nakadomarin A; along with the rest of the manzamine alkaloids it's been pretty popular over the last decade and I think that the field is still waiting for a 'final' synthesis. With potent cytotoxic, antibacterial and anti-microbial activity nakadomarin might be a little more exciting that the average natural product in terms of biological profile, but I suspect it’s the alluring structure and that unusual juxtaposition of small, medium and large rings that keeps synthetic chemists coming back for more. Certainly enough well-known groups have spent published work relating its synthesis. The double bonds in the two largest rings are just begging for an RCM-based approached, but it turns out (as with manzamine A), that this strategy is not as easy as it looks on paper. In fact, back in 2011 when I was considering a blog post on the (then) latest synthesis by Zhai, I made this graphic to illustrate the flaws with disconnection.[1] It might be a little dated now:[2]
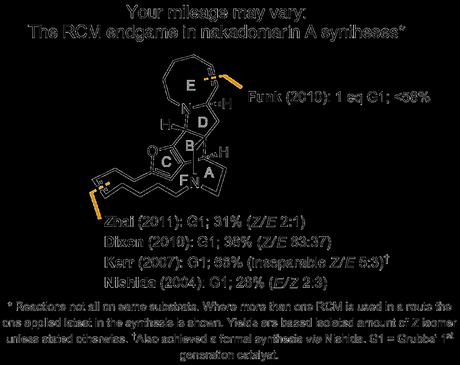
Evans decided to avoid opening that particular Pandora’s box and instead make both these potentially troubling rings as early as possible, breaking the molecule into two fragments with one larger ring in each. The two components were then to be united in a Lewis-acid mediated formal [4 + 2] reaction as shown below.[2] The group was pretty sure that the one existing stereocentre on the azocine ring junction would limit the approach of this pseudo-dienophilic component to one of two possible trajectories. It was hoped that the tendency of carbonyl dipoles to oppose one another—like in the famous Evans Aldol reaction—would cause the desired (bottom) approach to be somewhat more favoured.
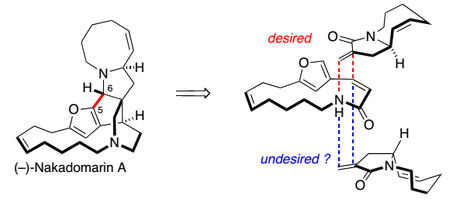
Starting with the preparation of the largest ring first, the group began with the iodination of 3-furfural. Although this is actually a literature procedure, I thought I’d include it because the temporary protection of the aldehyde by addition of lithium morpholide is quite a neat, yet uncommon tactic.[4] Next, the aldehyde was protected only slightly more permanently as the dimethyl acetal, allowing the newly installed iodide to be coupled Heck-style with allyl alcohol. A duo of Z-selective olefinations was then used to install the two halves of the large ring; the first was an ordinary unstabilised Wittig reaction, followed by acetal cleavage; this was swiftly followed a Still-Genari-type HWE reaction, carried out under strongly dissociating conditions for maximum Z-selectivity.[5] Finally, ester hydrolysis, Boc-deprotection and macrolactonisation with HBTU completed the synthesis of the coupling partner.
The pseudo-dienophilic component was prepared in just four steps, starting with a Zn-mediated allylation rendered asymmetric using Ellman’s handy chiral tert-butylsulfinamide methodology. Rather than using a preformed allylzinc reagent, the reaction was carried out under Barbier-type conditions, where Zinc dust was simply added to a mixture of the two starting materials. This initially resulted in rather low yields, due to the formation of N-allylated side products; however, this side-reaction could be suppressed by the addition of one equivalent of water (to protonate the Zinc(II) amide intermediate). Next. removal of the sulfinimide chiral auxiliary under acidic conditions, followed by treatment with sodium hydroxide resulted in the formation of the α-methylene-γ-lactam in good yield. Finally, construction of the azocine ring was achieved by alkylation with 1-iodo-5-hexene followed by treatment with Grubbs’ 1st generation catalyst to deliver the required bicyclic lactam.
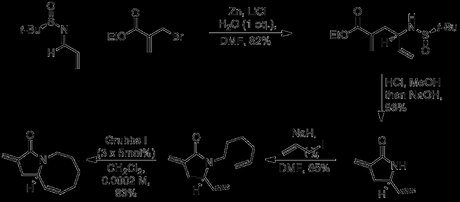
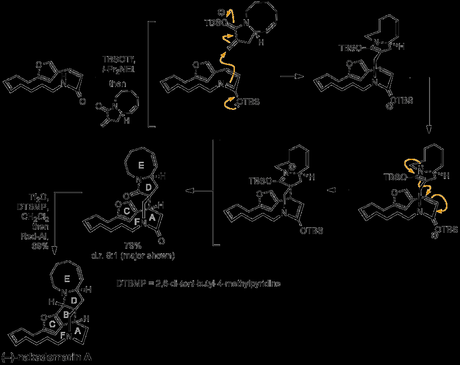
Now it was time for the group to investigate the key step: the Lewis acid mediated cascade reaction that would unite the two fragments synthesised so far. After some optimisation, it was found that reaction proceeded best when a solution of the bicyclic lactam was slowly added to a mixture of the macrocyclic lactam, TBS and Hünig’s base. Two equivalents of TBSOTf were found to be optimal, and triflic acid alone did not effect the reaction, implying that silylation is likely to be involved in the mechanism. The authors suggest a step-wise mechanism rather than a concerted [4 + 2] process, involving 1,4-attack of the silyl imidate formed from the macrolactam on an activated form of the exo-methylene of the bicyclic lactam, followed by cyclisation via a very similar mechanism. As well as an impressively high yield, a very reasonable diastereoselectivity (9:1) was observed (in favour of the desired product), presumably due to the tendency of the amide dipoles to oppose each other as the molecules approach each other. Now, all that remained for the completion of the synthesis was reduction of the six-membered lactam (in the presence of the five membered lactam!), followed by closure of the final ring. Perhaps unsurprisingly, this proved quite challenging, but could eventually be achieved by selective methylation of the six-membered lactam with Meerwein’s salt, followed by reduction of the resulting imidate with sodium borohydride; the final cyclisation could then be carried out by treatment with triflic anhydride, followed by reduction with sodium cyanoborohydride. However, after some further optimisation, this sequence could be performed in one pot from the formal [4 + 2] product; excess triflic anhydride was added first, effecting the cyclisation and activating the remaining amide, and a double reduction with Red-Al then gave the product. Awesome work!
Tangential Information
1. In case it's not clear, not all of the groups represented in this graphic performed the RCM on the same substrates, although they did use it to form the bonds marked. This is why the yields and E/Z ratios show the variation they do. Although a lot of information is lost in summarising so many different syntheses with just one structure, I just wanted to show you guys how low the yields are for this step!
2. I should add that this problem has just this year been solved by Shrock (along with a little help from Hoveyda and Dixon). You can check out the all-star paper here: Chem. Eur. J., 2013, 19, 2726. A 90% yield of 97% Z material can’t be sniffed at, although the catalyst isn’t exactly off-the-shelf.
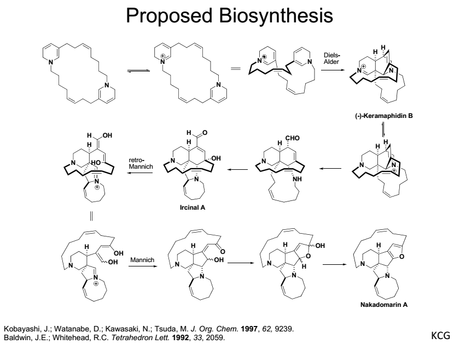
3. Not that this is thought to be biomimetic. The biosynthesis of this family, originally proposed by Baldwin and Whitehead (as far as I know), is quite well known. Here's a slide on it that I stole from a Denmark group talk:
4. For some reason, this loosely related trick use by Comins in his camptothesis synthesis has come to mind: Org. Lett., 2001, 3, 4255.
5. I was going to cite a few Gennari/Heathcock papers in support of this, but it might easier to see the neat Myers Chem 215 summary here.
